The fight goes on to rid the world of the COVID-19 coronavirus. And one Canadian company is on the forefront of the battle.

And plans to help win it.
Algernon Pharmaceuticals Inc. (AGN) (
CSE.AGN,
OTCQB:AGNPF,
Forum), a clinical stage pharmaceutical development company, just announced that it has received positive feedback from the U.S. Food and Drug Administration (FDA) regarding the Company’s plans to conduct a phase 2 COVID-19 clinical trial using its repurposed drug NP-120 (
Ifenprodil).
As part of AGN’s lead compound in its Idiopathic Pulmonary Fibrosis (IPF) research program, Ifenprodil is an orally delivered small molecule which was originally developed by global healthcare giant
Sanofi to treat peripheral circulatory disorders. Algernon has conducted two independent studies showing that Ifenprodil outperformed the world’s leading two treatments for IPF – Nintedanib and Pirfenidone in a recent pre-clinical in vivo animal study, reducing fibrosis by 56 percent with statistical significance.
What’s exciting news for investors is how a Chinese research team came to investigate Ifenprodil as a potential treatment for COVID-19.
They took human lung cells in an in-vitro experiment and conducted an RNAi interference assay randomly knocking out some 19,000 genes. Then, they exposed the cells to H5N1 (a lethal flu with a 50% mortality rate) and after the experiment ended, harvested the cells that survived. They then looked at the genes in the surviving cells and identified which genes were affected. Then they screened thousands of compounds from the drug bank database to see which of those drugs
(image via Algernon Pharmaceuticals Inc.)
effected the same genes that helped protect the lung cells against H5N1 and Ifenprodil was one of the key drugs.
The US FDA announcement follows news from the clinical stage, where Health Canada also gave positive feedback over its Phase 2 COVID-19 clinical trial. AGN also recently reported that a regulatory submission had been made to the Ministry of Food and Drug Safety in South Korea for an investigator-led COVID-19 study with Ifenprodil.
Once the trial has been approved, the Company, along with its lead Asia-Pacific CRO Novotech and the physician-investigators, will work to enroll patients and begin the study as soon as possible.
More About NP-120 (Ifenprodil)
NP-120, or Ifenprodil, is an N-methyl-D-aspartate (NMDA) receptor antagonist specifically targeting the NMDA-type subunit 2B (Glu2NB). Ifenprodil prevents
glutamate signaling. The NMDA receptor is found on many tissues including lung cells and T-cells.
The Company says Ifenprodil can reduce the infiltration of neutrophils and T-cells into the lungs where they can each release glutamate and cytokines respectively. The latter can result in the highly problematic cytokine storm that contributes to the loss of lung function and ultimately death as has been reported in COVID-19 infected patients.
A Canadian scientist by the name of Dr. Mark Williams demonstrated a keen eye for seeing things others didn’t. As a result, he identified the key receptor that the drug targets in the brain and noticed that the same receptors – NMDA – are also present in human lung tissue. He had a theory and convinced a company to invest in his idea. The Company was Algernon.
The data was substantial –
Ifenprodil did a better job at reducing fibrosis in a serious lung disease called idiopathic pulmonary fibrosis (IPF), beating the world’s two leading human treatments sold by Roche and Boehringer Ingelheim in the same animal study.
And investors and company shareholders – both institutional and retail – are acutely aware of the game-changing science and technology this potentially offers to treat and attack the global coronavirus pandemic.
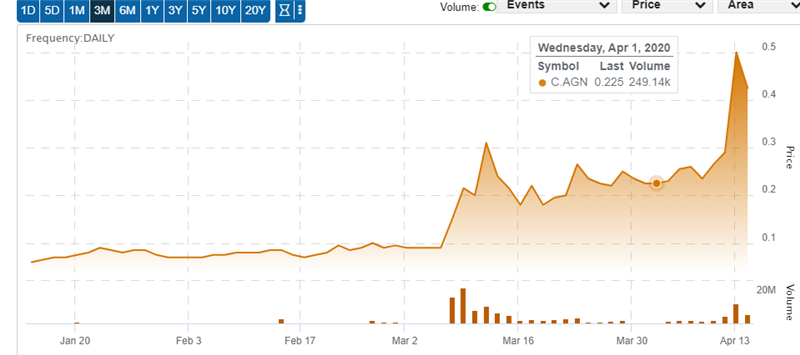 (Click image to direct to chart)
(Click image to direct to chart)
This
announcement from Algernon Pharmaceuticals signals a major step forward in the Company’s first-mover drug repurposing strategy and has been bolstered by additional FDA approval of a toxicology program for a new intravenous Ifenprodil formulation, where a single animal 30-day study would be acceptable.
FULL DISSCLOSURE: This is a paid article produced by Stockhouse Publishing.