TUCSON, Ariz., May 2, 2017 /PRNewswire/ -- Roche (SIX: RO,
ROG; OTCQX:RHHBY) today announced approval of the VENTANA PD-L1 (SP263) Assay by the US Food and Drug Administration (FDA) as a
complementary diagnostic3 to provide PD-L1 status for patients with locally advanced or metastatic urothelial
carcinoma (mUC)4 who are being considered for treatment with the FDA-approved anti-PD-L1 immunotherapy IMFINZI™
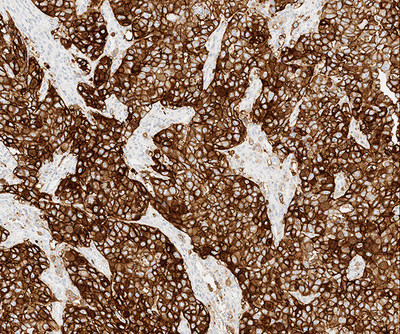
(durvalumab, AstraZeneca). The test evaluates patient PD-L1 status using both tumor and immune cell staining and scoring within
the tumor microenvironment, providing clinicians with information that may guide treatment decisions. PD-L1 is a protein involved
in the suppression of the immune system, which can impact the body's ability to fight cancer. Understanding the expression of
PD-L1 in tumors can help identify patients most likely to benefit from immunotherapy.
It is estimated that in 2017, approximately 79,000 Americans will be diagnosed with bladder cancer and almost 17,000 will die
from this disease. Men are three to four times more likely than women to suffer from this cancer.1
"Urothelial carcinoma is an area of significant unmet medical need," said Ann Costello, Head of
Roche Tissue Diagnostics. "We are very pleased the VENTANA PD-L1 (SP263) Assay has received FDA approval as it will serve as a
powerful tool to help inform physicians about appropriate treatment options for their patients."
Roche continues to pursue regulatory approval for the VENTANA PD-L1 (SP263) Assay in other cancer indications in the US and in
other geographies. This collaboration with AstraZeneca demonstrates Roche's continued commitment to personalized medicine through
innovative diagnostic solutions.
About VENTANA PD-L1 (SP263) Assay
VENTANA PD-L1 (SP263) Assay is intended for the qualitative detection of the Programmed Death Ligand (PD-L1) in a
growing number of cancer indications. The assay is available in the US for use on the BenchMark ULTRA instrument.
The use of the VENTANA PD-L1 (SP263) Assay for PD-L1 expression testing in either tumor or immune cell membranes in urothelial
carcinoma may be a useful tool to help determine the likelihood of responding to IMFINZI™ (durvalumab) but is not required for
use of IMFINZI.
Find more information and available testing locations at www. pdl1ihc.com.
About durvalumab
IMFINZI™ (durvalumab) is a human monoclonal antibody directed against PD-L1. Durvalumab is also being studied in the
first-line treatment of patients with unresectable and metastatic bladder cancer as a monotherapy and in combination with
tremelimumab, a checkpoint inhibitor that targets CTLA-4, as part of the DANUBE Phase III trial, which enrolled its first patient
during the fourth quarter of 2015. Additional clinical trials are ongoing to investigate durvalumab as monotherapy or in
combination with tremelimumab in non-small cell lung cancer, head and neck squamous cell carcinoma, bladder, hepatocellular
carcinoma and blood cancers. IMFINZI is a trademark of the AstraZeneca group of companies and is manufactured and distributed by
AstraZeneca.
For more information on IMFINZITM (durvalumab), visit www.imfinzi.com
About Roche
Roche is a global pioneer in pharmaceuticals and diagnostics focused on advancing science to improve people's lives.
The combined strengths of pharmaceuticals and diagnostics under one roof have made Roche the leader in personalised healthcare –
a strategy that aims to fit the right treatment to each patient in the best way possible.
Roche is the world's largest biotech company, with truly differentiated medicines in oncology, immunology, infectious
diseases, ophthalmology and diseases of the central nervous system. Roche is also the world leader in in vitro diagnostics and
tissue-based cancer diagnostics, and a frontrunner in diabetes management.
Founded in 1896, Roche continues to search for better ways to prevent, diagnose and treat diseases and make a sustainable
contribution to society. The company also aims for improving patient access to medical innovations by working with all relevant
stakeholders. Twenty-nine medicines developed by Roche are included in the World Health Organization Model Lists of Essential
Medicines, among them life-saving antibiotics, antimalarials and cancer medicines. Roche has been recognised as the Group Leader
in sustainability within the Pharmaceuticals, Biotechnology & Life Sciences Industry eight years in a row by the Dow Jones
Sustainability Indices (DJSI).
The Roche Group, headquartered in Basel, Switzerland, is active in over 100 countries and in
2016 employed more than 94,000 people worldwide. In 2016, Roche invested CHF 9.9 billion in R&D
and posted sales of CHF 50.6 billion. Genentech, in the United
States, is a wholly owned member of the Roche Group. Roche is the majority shareholder in Chugai Pharmaceutical,
Japan. For more information, please visit www.roche.com.
VENTANA and BENCHMARK are trademarks of Roche. Other product names and trademarks are the property of their respective
owners.
Roche Tissue Diagnostics Media Relations
Gabrielle Fimbres
Sr. Manager, External Communications
Phone: +1 520.222.4573
Email: gabrielle.fimbres@roche.com
1American Cancer Society, 2017 bladder cancer statistics.
2This product is intended for in vitro diagnostic (IVD) use.
3Complementary diagnostic is a test that aids in the benefit-risk decision making about use of the therapeutic
product. (Reena Phillips. Developing Diagnostics: Perspective From the FDA. ASCO 2016
presentation, http://meetinglibrary.asco.org/content/51097?media=vm )
4Metastatic urothelial carcinoma (mUC) is also known as transitional cell carcinoma (TCC) of the urinary tract or
urothelial bladder cancer. The majority of urothelial tumors arise in the bladder with the remainder originating in the renal
pelvis, urethra or ureter.
To view the original version on PR Newswire, visit:http://www.prnewswire.com/news-releases/roche-receives-fda-approval-for-complementary-pd-l1-sp263-biomarker-test-in-urothelial-carcinoma-300449923.html
SOURCE Roche