As a general rule, the most successful man in life is the man who has the best information
Endoscopy is often used in the prevention, early detection, diagnosis, staging, and treatment of cancer.
X-rays and CT scans can show physical changes within the body and give information about the size, shape, and location of the changes. Endoscopes show details like color and surface texture allowing doctors to see exactly what’s going on.
If you go to a doctor exhibiting certain symptoms, endoscopy could be used to find the cause:
- Long-term hoarseness - Laryngoscopy to look at the vocal cords
- Trouble swallowing - Upper endoscopy
- Anemia (low red blood cell counts) with an unknown cause - Colonoscopy
- Blood in stool - Colonoscopy
Colonoscopy and sigmoidoscopy are used to look for cancer in people who have no symptoms by screening for colon and rectal cancer. They also help prevent cancer by finding polyps (growths in the bowels) that could become cancer. Thoracoscopy and laparoscopy can be used to find out if cancer has spread into a patients thorax or abdomen.
Most endoscopes can, with an attached tool, take small tissue samples – called a biopsy. Instruments passed through an endoscope can also be used to cut out growths, a cautery or laser can be used to burn or vaporize them.
Minimally invasive surgery
Many types of endoscopic tools have been developed to let doctors perform minimally invasive surgery.
When endoscopy is used for the abdomen it is called laparoscopic surgery. Instead of one long incision several small cuts are made - usually in the chest or abdomen. A video endoscope – a thoracoscope or laparoscope – is put through one of the holes so that the surgeon can see inside during the operation.
This type of surgery, called video-assisted thoracoscopic surgery or VATS, can also be used to treat small lung cancers. It can also be used for the colon (laparoscopic colectomy), prostate (laparoscopic radical prostatectomy), and some other organs.
Patients, physicians, providers, and payers have wholeheartedly embraced minimally invasive therapy for many reasons:
- Minimally invasive therapy obviates the need for major open-surgery procedures.
- Minimally invasive therapy produces much less of the sequelae (a condition that is the consequence of a previous disease or injury) of open surgery procedures.
- Minimally invasive therapy leaves minute scars versus open-surgery procedures.
- Minimally invasive therapy results in shorter hospital stays and reduced outpatient treatments.
- Minimally invasive therapy results in a much more rapid return to normal activity.
- Reductions in length of hospitalization and the ability to return to work much sooner are economically attractive.
The demand for endoscopy as a tool in
cancer detection has been increasing significantly because of the growing preference for minimally invasive surgeries, which reduce patients’ pain, speed recovery and reduce the overall costs to the healthcare system.
The endoscope is the most important weapon in the minimally invasive therapy arsenal.
Other factors driving the growth of global endoscopy include:
- Aging population
- Increasing prevalence of diseases that require endoscopy procedures
Bladder cancer
Fact; Advances in bladder cancer treatments, let alone a breakthrough, have been slow in coming - no new products have been developed and Urologists have been using the same diagnosis and treatment methods for decades.
According to the National Cancer Institute (NCI) bladder cancer is the sixth most common cancer in the United States and the third most common cancer in men, with over 72,000 new cases diagnosed annually (380,000 worldwide). It is estimated that approximately 577,400 people are currently living with bladder cancer in the United States, generating over 1,500,000 physician consultations per year, and that approximately 16,000 individuals will died from the disease in 2015.
Bladder cancer facts:
- Low grade non-muscle bladder cancer has a reoccurrence rate of 40%. High grade non-muscle bladder cancer has a reoccurrence rate of 70%. The average reoccurrence rate for this type of cancer is nearly 50%, which is one of the highest reoccurrence rates of all cancers.
- Bladder cancer is the most expensive cancer to treat in the US.
- Bladder cancer represents 4.6% of all new cancer cases in the U.S.
- In 2016, it is estimated that there will be 76,960 new cases of bladder cancer and an estimated 16,390 people will die of this disease.
- Age: Seniors are at the highest risk of developing bladder cancer.
- Sex: Men are three times more likely than women to have bladder cancer.
- Race: Whites have a much higher risk of developing bladder cancer than other races.
Bladder cancer is generally identified in the clinic by a procedure called cystoscopy, an endoscopy in the bladder.
When detected early, bladder cancer can be treated successfully. Initial treatment of bladder cancer is based on a tumor’s clinical stage, determined by how deep the tumor is thought to have grown into the bladder wall, and whether or not it has spread beyond the bladder. Other factors, such as the size and grade of the tumor, may also affect treatment options.
Unfortunately, bladder cancer has a very high rate of recurrence, one of the highest among the cancers. Because of the high risk of recurrence, patients who have been treated for bladder cancer undergo follow-up endoscopy every 3-6 months. For the rest of the patient’s life, a cystoscopy on a quarterly, semi-annual or annual basis is essential.
It is estimated that over 4 million cystoscopies are performed each year and approximately US$4B is spent on bladder cancer surveillance annually.
Bladder cancer is the most expensive cancer to treat over the lifetime of a patient.
The majority (about 70%) of bladder cancers are superficial meaning they are only in the lining of the bladder. However, if left undiagnosed and untreated, these cells can invade the muscle wall which could require complete removal of the bladder - a radical cystectomy.
It is important that cystoscopy imaging be both:
- Highly sensitive by being able to detect subtle cancer
- Specific meaning able to distinguish between benign and cancerous tumors
These two attributes enable surgeons to remove cancerous tissue at an early stage.
While cystoscopy is the “gold standard” for a routine surveillance exam it’s well known that standard cystoscopy has insufficient sensitivity and specificity. This is particularly true for flat appearing cancers that blend in with the bladder and may be confused with inflammation.
Various experimental studies demonstrate a 4-10% rate of missed bladder tumors by conventional cystoscopy – carcinoma in situ bladder cancer (CIS) 4 is a high grade cancerous lesion, often diffuse and difficult to visualize it can be a very aggressive form of the disease.
There is an important need to improve the ability of endoscopy to 1. identify suspicious bladder lesions without missing any cancers and 2. to characterize bladder lesions as benign or malignant with high accuracy.
The most common symptom of bladder cancer is blood in the urine (hematuria) with little or no pain. It should ALWAYS be assessed by a physician.
Let’s look at current endoscope technology as it involves cancer.
White Light and Cancer
White light is the standard convention and it’s what’s commercially available in all endoscope devices manufactured today. White light has been utilized in endoscopes for decades to guide the physician and surgeon so they can see cancerous growths that protrude above an organs surface, do biopsies and remove suspicious growths.
White light is comprised of energy in the form of electromagnetic radiation that vibrates at many different wavelengths. Wavelengths between 390 nm and 780 nm are visible to the human eye and produce the different colors of the spectrum.
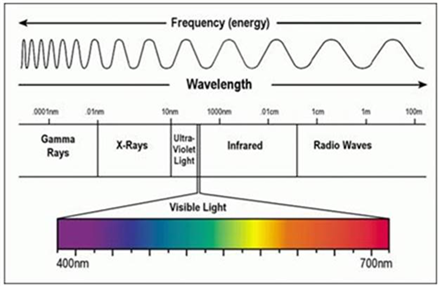
White light has limitations in visualizing certain cancer types because:
- White light cannot pass through tissue or blood
- White light cannot illuminate tumors beneath the skin surface.
- White light is not effective in visualizing the borders of the tumor to determine where it begins and ends (the margins), especially after the initial removal of the main mass. If the surgeon does not remove all the cancerous growth, and a few cancerous cells remain, the tumor can grow back and spread, or metastasize to other parts of the body
- Malignant and premalignant tumors that are flat or very small may look similar to normal tissues. As a result, a physician may not be able to identify some aggressive cancers. In order to be safe, they may collect random and repeat biopsies as the only possible way to ensure that cancer is not missed in high-risk patients
To summarize, white light has visualization limitations for all cancer types because white light cannot pass through tissue or blood and cannot illuminate tumors beneath the skin surface. White light is often not effective in visualizing the borders or margins of the tumor to determine where it starts and ends especially after the initial removal of the main mass.
Blue Light
Because of the limitations with using white light for visualizing cancers, various companies have begun using blue light (white light with blue filter) in conjunction with chemical tumor targeting/imaging agents. This improved technology introduces a red fluorescence to the tumor and has improved the ability to visualize cancers and margins.

Unfortunately these chemical agents can cause various adverse effects - including anaphylaxis shock and hypersensitivity reactions - with repeated usage at the high doses currently required for visualization. The FDA has limited use of these chemical tumor targeting/imaging agents to just once per patient. Doctors and surgeons cannot repeatedly examine a patient using these chemical imaging agents. This creates a huge problem treating patients with multiple tumors and those with recurrent tumors.
Red Light
Red light requires specialized laser light sources, ultrasensitive cameras and a unique optical design. Currently no commercial instruments are available using red light.
The Unmet Need
What is acutely needed right now, across the entire spectrum of the endoscope imaging space/sector, is an ultrasensitive system that uses white light while simultaneously using other wavelengths of light to visualize all tumors, and one that allows multiple usage in patents.
Future endoscopes should also have more advanced cancer detection technologies so that ultimately no chemical imaging agents would be necessary.
These future tools should provide ultrasensitive and advanced imaging capabilities and the system should be capable of being used in a physician’s office or clinic for diagnosis, in the operating room and ambulatory surgical center.
Imagin Medical
Imagin Medical (CSE: IME) (OTC: IMEXF) is a biotechnology company founded to commercialize an ultrasensitive, next generation imaging technology for extremely accurate visualization of cancers.
Invented by Dr. Stavros Demos at the Lawrence Livermore National Laboratory (LLNL), this combination optical/laser technology uses white light and near-infrared fluorescence to accurately visualize and detect cancer. To validate this technology, Dr. Demos worked for over five years in collaboration with the UC Davis Comprehensive Cancer Center and Dr. Ralph deVere White, one of the world’s leading authorities on bladder cancer.
Imagin now holds the exclusive license to this intellectual property. The Company has entered into an agreement with UC Davis Comprehensive Cancer Center and the University of Rochester Laboratory for Laser Energetics in New York, where Dr. Demos will support the company’s effort to complete development.
IME believes it will radically improve the way physicians detect cancer through the use of endoscopes to image and visualize cancer and/or cancer markers.
Imagin is developing systems that will potentially enable physicians to accurately detect cancer with minimal use of, or no toxic contrast agent, and remove all tumor tissues and cancerous cells completely during the first procedure. This technology, after having being proven successful in the operating room, will greatly reduce the chances of cancer recurrence and allow safe, multiple follow-up screenings for patients that can be performed during routine monitoring by physicians in an outpatient setting.
Imagin Medical’s advanced ultrasensitive imaging technology is based upon improved optical designs and components, and advanced light sensors. The results are:
- Increased sensitivity and specificity for the detection of cancers and even premalignant lesions.
- A potential decrease in cancer recurrence due to the ability to completely remove tumor tissues along with the cancerous cells in the margins.
- A significant commercial advantage to Imagin’s imaging technology because of its adaptability to all endoscopes that are currently on the market.
- Easy adoption of Imagin’s two ultrasensitive imaging designs for use in multiple other applications where endoscopy imaging is currently utilized.
Imagin Medical’s ultrasensitive i/Blue and i/Red Imaging Systems use white light in conjunction with the Company’s unique fluorescent wavelengths to radically improve the physicians ability to visualize (detect) cancer. This technology is estimated to increase sensitivity to an estimated 5 orders of magnitude (100,000 x) more than white light alone.
Beginning in 2010, the FDA approved blue laser light to be used with various imaging agents, but only on a one-time per patient basis because of potentially dangerous side effects. This limitation generally restricts physicians use of imaging agents for the O.R. so they can take advantage of this one-time opportunity to operate immediately, if and when cancer is found.
The early detection and assessment of bladder cancer relies on the accuracy of imaging technology via endoscopes. Conventional endoscopes use white light and produce less-than-adequate images to view during surgical procedures that can result in misdiagnosis or incomplete removal of cancer cells, leading to recurrence. These chemical imaging agents improve the quality of the image and the ability to differentiate healthy cells from cancerous, yet they require a one-hour prep time which can be logistically impractical and costly for the Operating Room.
Imagin Medical is developing the i/Blue™ Imaging System to help detect bladder cancer and reduce its recurrence by dramatically improving the urologist’s ability to visualize, identify and remove cancerous cells. This advanced combination of optical/light sensor technology, white light and near-infrared fluorescence delivers superior images in less than 15 minutes vs. the one hour required by conventional systems. The time savings will increase the efficiency of the Operating Room and reduce healthcare costs by potentially enabling the procedure to be performed in the less expensive physician’s office for follow-up exams.
Additionally, physicians using today’s standard blue light need to switch back and forth between the white light and blue light images, which is not necessary with the i/Blue Imaging System that blends both lights into one image.
The i/Red Imaging System, the Company’s next advancement, uses a unique red laser light to illuminate the cancer and requires no imaging agents at all. This breakthrough totally disruptive technology uses only the fluorescence produced by the body and tumor itself.
The i/Red Imaging System will dramatically broaden the market to all cancer specialists using any type of scopes.
Competitive Advantages
Imagin Medical’s advanced ultrasensitive imaging technology will lead the marketplace in illumination of cancerous cells and provide an improved surgical outcome as a result of an improved detection and resection, which will lead to more adequate patient management and follow-up.
Competitive Advantages:
- Can be seamlessly adapted to any type of endoscopic or other type of imaging device commercially available.
- Based upon improved optical designs and components, and advanced light sensors. The result is increased sensitivity and specificity for the detection of cancers, including premalignant lesions.
- Will decrease bladder cancer recurrence due to the ability to completely remove tumor tissues along the margins.
- Will add significant ease-of-use for the surgeon and staff in the O.R. because of the dual imaging capability.
- Imagin Medical has an ultrasensitive red light endoscopy system that requires no chemical imaging agents.
- Because there is no need for chemical imaging agents, this system can be used in a physician’s office or clinic for cystoscopy (diagnosis), and in the operating room (O.R.) or ambulatory surgical center for tumor removal or resection.
- Imagin Medical has patents covering these imaging systems that are estimated to be at least 100,000 times more sensitive in tumor detection than any other devices currently in the marketplace.
- Provide improved detection of cancer superior to what is currently in the market place.
- The white light and fluorescence images are recorded and displayed simultaneously providing an effective real time navigation tool that can be farther enhanced using processing (such as overlapping and pseudo-coloring) of the two principal images.
An Execution Play
The company’s products are based on the
technology invented by Dr. Stavros Demos at the Lawrence Livermore National Laboratory (LLNL). Dr. Demos worked in collaboration with the UC Davis Comprehensive Cancer Center and Dr. Ralph deVere White, one of the world’s leading authorities on bladder cancer, for more than five years to prove feasibility. Imagin has moved the final stage of development to the University of Rochester Laboratory for Laser Energetics, where Imagin’s engineering team, with the support of Dr. Demos, will complete additional clinical evaluations and prepare for FDA submission.
Conclusion
Fact; Current cystoscopy has well-documented problems associated with the limited ability to distinguish cancer from normal tissue.
Imagin’s strategy is to set a new “standard of care” in detecting bladder and other cancers by introducing game-changing disruptive advances in endoscope technology with its i/Blue and i/Red combination optical/laser systems.
Imagin Medical CSE - IME, and their significantly improved endoscope technology, need to be on your radar screen.
Is advanced endoscopy on your screen?
If not, it should be.
Richard (Rick) Mills
***
Richard owns shares of Imagin Medical (CSE: IME) (OTC: IMEXF)
Richard lives with his family on a 160 acre ranch in northern British Columbia and is the owner of Aheadoftheherd.com.
Richard’s articles have been published on over 400 websites, including:
WallStreetJournal, USAToday, NationalPost, Lewrockwell, MontrealGazette, VancouverSun, CBSnews, HuffingtonPost, Beforeitsnews, Londonthenews, Wealthwire, CalgaryHerald, Forbes, Dallasnews, SGTreport, Vantagewire, Indiatimes, Ninemsn, Ibtimes, Businessweek, HongKongHerald, Moneytalks, SeekingAlpha, BusinessInsider, Investing.com, MSN.com and the Association of Mining Analysts.
Sign up for Ahead Of The Herd’s free
highly acclaimed newsletter.
***
Legal Notice / Disclaimer
This document is not and should not be construed as an offer to sell or the solicitation of an offer to purchase or subscribe for any investment.
Richard Mills has based this document on information obtained from sources he believes to be reliable but which has not been independently verified.
Richard Mills makes no guarantee, representation or warranty and accepts no responsibility or liability as to its accuracy or completeness. Expressions of opinion are those of Richard Mills only and are subject to change without notice.
Richard Mills assumes no warranty, liability or guarantee for the current relevance, correctness or completeness of any information provided within this Report and will not be held liable for the consequence of reliance upon any opinion or statement contained herein or any omission.
Furthermore, I, Richard Mills, assume no liability for any direct or indirect loss or damage or, in particular, for lost profit, which you may incur as a result of the use and existence of the information provided within this Report.