RE:RE:Dr. Kamat Speaks with ImmunityBio's Dr. Soon-ShiongI would also note that that 58% CR at 12 months was not the CR percentage of the 77 patient trial population. It was the percentage of the 48 out of 77 patients (62% CR at any time) who were still CR at 12 months.
28 out of 77 patients were CR at 12 months = 36% CR
They still exceed the IBCG recommendation - but only by 6% - not 28% as they made it sound.
DJDawg wrote: A few thoughts about this very interesting interview.
Dr. Soon-Shiong is very excited by how the anktiva got a 58% rate at 12m vs the IBCG recommendation of 30% or greater. I looked up the IBCG document and the 30% he is mentioning is recurrence free rate. This is different than the CRR that we use and talk about most with TLT. The math for TLT is a recurrence free rate is 65% vs the 58% for anktiva.
They talk about getting BCG from india. The talk about clinical trials to get that version of BCG approved. That doesn't not seem like a quick and easy step at all. Until then, they are anchored to the supplies of available BCG which is low.
Finally, when the audience of uro-oncologist was surveyed at a very recent cancer meeting the response was no where what you would imagine if you read Dr. Soon-Shiong's enthusiastic comments in the interview. You can see from the image below, that, for the scenario posed, 4% would use anktiva.
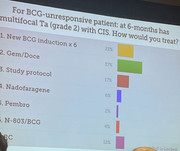
https://i.postimg.cc/JntDDzyy/IMG-8466.jpg
Most would use gem/doc which has been around longer. It uses 6 treatment to start, one week apart. That is a longer procedure with two drugs instilled. Then it goes to monthly for 10 months. Not every 3 m like many meds. Finally, if you are a responder it is MONTHLY as maintenance for years to follow.