Fred ... This is Oncolytic's interim data @12-month for the BCG-unresponsive market:
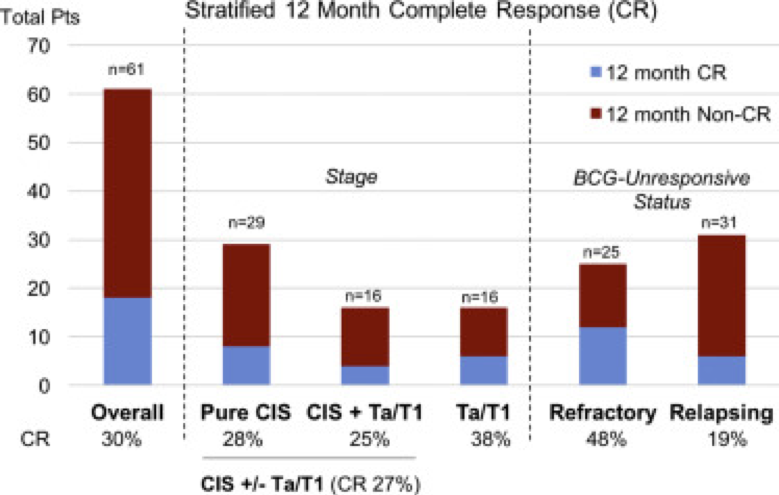
Overall 12 month CR rate was 30%. CIS-containing tumors had 27% 12 month CR (n=45) while pure Ta/T1 had 38% (n=16). No patients with T1/CIS or Ta/CIS had 12 month CR (n=6). When looking at BCG response: BCG-refractory patients had 48% (n=25) 12 month CR, BCG-relapsed 19% (n=31), unknown 0% (n=5). Following trial entry, 10 patients underwent cystectomy, of which 6 patients had muscle invasive disease.
In terms of tolerability, all 12 month treatment related adverse events (AEs) were Grade 1-3; immunologic AEs included influenza type illness (7%), fatigue (4%), and chills (1%). Five deaths were secondary to progressive urothelial carcinoma, esophageal carcinoma, lung carcinoma, and cardiac disease.
Overall, tolerability remained quite good. In general, the intervention appears to be less effective in CIS patients. The summary of response rates stratified by initial histology and BCG response were presented in the following table:
It's more recent and differ a bit from the 2016 link you posted about 3 days ago:
https://abstracts.mirrorsmed.org/abstracts/phase-iii-canon-study-oncolytic-immunotherapy-treatment-non-muscle-invasive-bladder-nmibc that stated this:
Phase I/II CANON study: Oncolytic immunotherapy for the treatment of Non-Muscle Invasive Bladder (NMIBC) Cancer using Intravesical Coxsackievirus A21 Mostafid Hugh University of Surrey, Guildford, UK Royal Surrey County Hospital Guildford Surrey UK
CAVATAKTM is a novel, bio-selected ICAM-1 targeted immunotherapeutic Coxsackievirus A21 (CVA21). Surface ICAM-1 is up-regulated on NMIBC. CVA21 displays potent oncolytic activity against in vitro cultures of NMIBC cancer cells and ex-vivo human bladder tumor. Combining CVA21 with mitocycin C (MMC) synergistically enhances viral replication by increasing expression levels of ICAM-1. In this two stage Phase I/II study, patients (pts) with NMIBC received neo-adjuvant CVA21 or low dose MMC plus CVA21 prior to surgical removal (TURBT).
The CANON study investigated the tolerance of escalating intravesicular (IV) doses of CVA21 in 16 first-line NMIBC cancer pts. Stage 1 Cohort 1 (n = 3) and Cohort 2 (n = 3), pts received a single CVA21 administration at 1 x 108 and 3 x 108 TCID50, respectively. In Cohort 3 (n = 3), pts received 2 doses of CVA21 at 3 x 108 TCID50. Stage 2: Cohort 1 (n = 3), pts received a single CVA21 dose of 3 x 108 TCID50 and Cohort 2 (n = 3) with 2 doses of CVA21 at 3 x 108 TCID50, both in combination with MMC (10mg). Cystoscopy photography was performed before and after treatment (tx). IV tx was followed by TURBT surgery after 8-11 days, with tissues analysed for CVA21 replication, apoptosis, evidence of viral-induced changes, immune cell infiltrates (multi-spectral imaging) and immune checkpoint molecules.
Data indicate tolerance of IV CVA21 tx. Serial cystoscopy identified viral-induced surface haemorrhage and immune inflammation of the tumor micro-environment. Virus replication within tumor was highlighted by detection of secondary viral load peaks in the urine. TURBT tissue analysis displayed marked tumor specific viral replication and evidence of viral-induced apoptotic cell death. NanoString analysis identified widespread increases in interferon (IFN), viral RNA and immune-checkpoint genes in CVA21-treated tissues compared to untreated historical controls. Furthermore, after exposure to CVA21 six of eleven patients studied had increased urinary levels of HMGB1, a critical chemokine involved in initiating and maintaining an immune response.
Clinical activity of CVA21 was demonstrated by evidence of complete tumor response, viral replication and notable signs of tumor inflammation. The observed up-regulation of IFN/immune checkpoint genes provides evidence for the generation of both strong local and systemic anti-tumor immune responses.