Updated Research Page on McFarland Labs WebsiteResearch Overview
The primary work of this lab is in the development of light-activated drugs that can be used to treat cancer. In this context the advantage of a photodrug is that it can impart spacial and temporal selectivity: the activity ceases when illumination is terminated. Consequently, such a treatment offers the promise of effective cancer treatment while avoiding the systemic complications often associated with other modalities.
Photodynamic therapy (PDT) and the relatively new field of photochemotherapy (PCT) are examples of such locally administered treatment, where a non-toxic prodrug — the photosensitizer (PS) — becomes activated by light to form reactive species that destroy tumor tissue. In the case of classical PDT, the cytotoxicity arises from singlet oxygen and/or other reactive oxygen species (ROS). In this way cancerous tissue is destroyed, but healthy tissue is spared, leading to superior clinical outcomes. Furthermore, we have very good evidence that our compounds elicit an immune response, leading to the suppression of metastases.
While PDT has shown remarkable efficacy against certain cancers, its widespread adoption has been limited by relatively few PSs having been approved as clinical agents. The lack of attention to the importance of the light dose protocol has also restricted progress; PSs have usually been developed in isolation from their end use, and the one-PS-for-all-applications approach has not produced clinical results. Long-lived photoexcited triplet states are the foundation of PDT. We use the rich and highly tunable photophysics and photochemistry of transition metal complexes that offer a variety of triplet state configurations that are not available to organic chromophores (which are exclusively π-π*). The low-spin d6 polypyridyl complexes of Ru(II) and Os(II) are of particular interest to us, having nearly 100% quantum yields for intersystem crossing to the triplet states that lead to photocytotoxicity. The distinct chemical reactivity of these states can be exploited to favor specific photophysical pathways not available to organic PSs, ultimately to elicit a desired biological response. Because these polypyridyl-type complexes are inherently modular, the design of the individual ligands gives fine-tuned control of the PS photochemistry and photophysics, which can lead to PSs tailored for specific cancer indications.
Our approach is tumor-centered and is inherently multidisciplinary: this group has consisted of students and researchers from chemistry, physics, biology, and biomedical engineering. We use the tools of synthetic chemistry, photophysics and photochemistry, microscopy, cell culture, and laser spectroscopy to take our treatment from the concept stage all the way to human trials. The strategy is sound; one of our compounds, TLD1433, is currently in a Phase II clinical trial for treating non-muscle invasive bladder cancer, with very encouraging preliminary results, and it has been designated fast track status by the FDA.
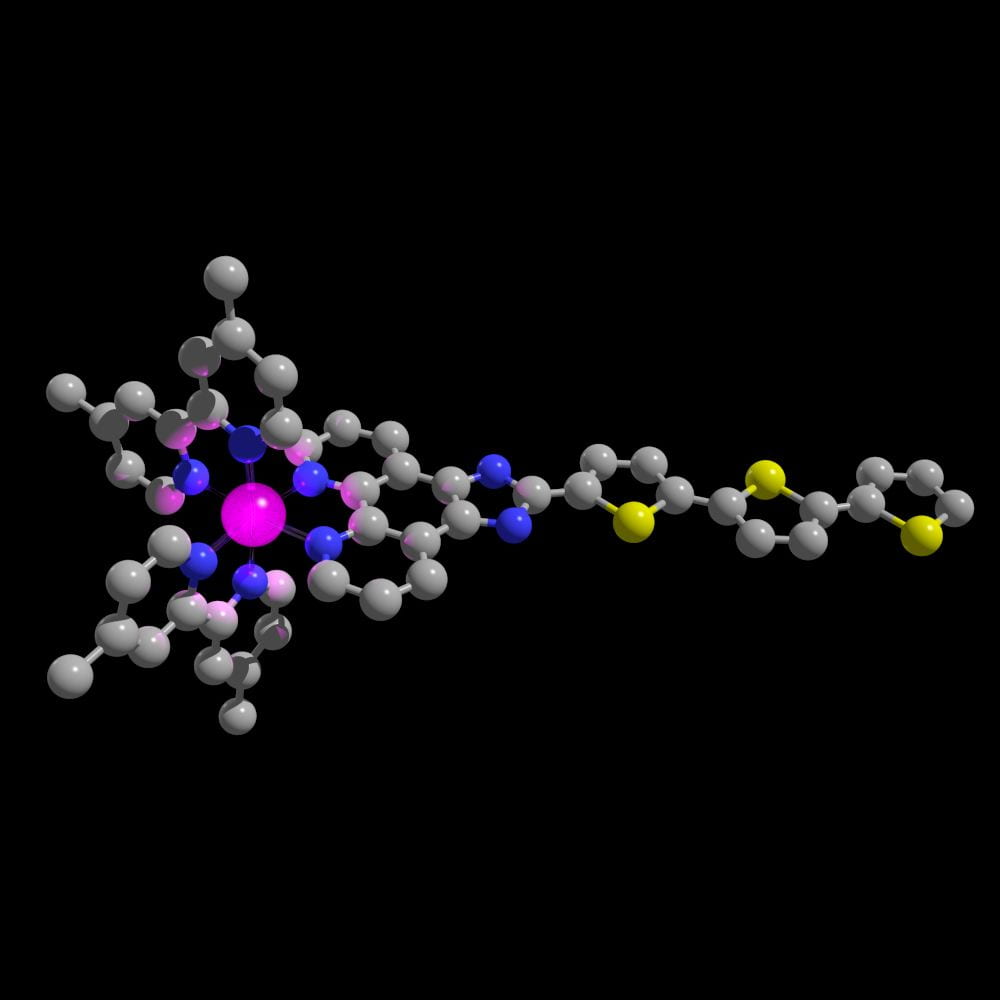 TLD1433, the first ruthenium-based photodrug to progress to human clinical trials.
TLD1433, the first ruthenium-based photodrug to progress to human clinical trials. 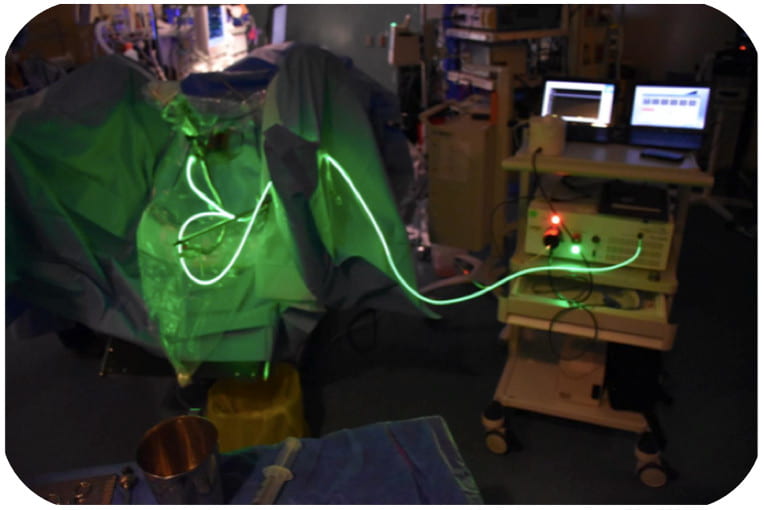 TLD1433 being used to treat a bladder cancer patient in a clinical setting using specialized light delivery apparatus.
TLD1433 being used to treat a bladder cancer patient in a clinical setting using specialized light delivery apparatus. 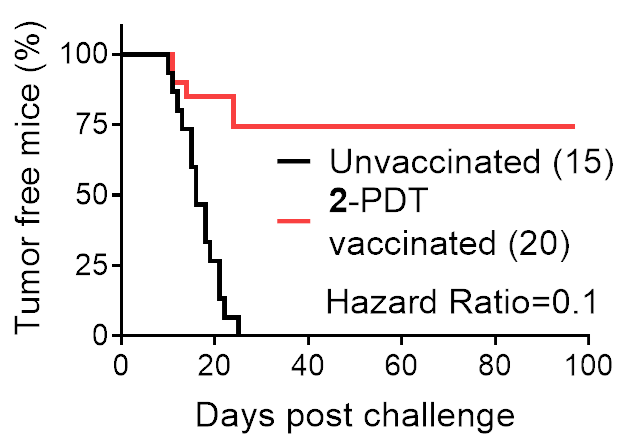 PDT-induced immunogenic protection against B16F10 melanoma in a mouse model, showing tumor-free survival curves of unvaccinated and vaccinated C57BL/6NCrl female mice. Published data.
PDT-induced immunogenic protection against B16F10 melanoma in a mouse model, showing tumor-free survival curves of unvaccinated and vaccinated C57BL/6NCrl female mice. Published data. Synthesis
Our laboratory maintains significant organic and inorganic synthetic capacity; we design and build the organic π-expansive pyridyl compounds to be used as ligands, and then assemble them into metal complexes for use as PSs. We use a variety of modern techniques to synthesize, purify, and characterize new generations of metal-based photosensitizers (PSs). We favor microwave reactors where possible to accelerate throughput. Purification often involves flash columns and size exclusion chromatography, with characterizion by techniques such as 1D and 2D NMR, high-performance liquid chromatography (HPLC), high-resolution mass spectrometry (ESI+ MS). The resulting purified complexes are then passed on for spectroscopic and photobiological examination as PDT/PCT agents.
Photochemistry and Photophysics
Polypyridyl complexes of Ruthenium(II) and Osmium(II) have constituted a useful platform for our PS sensitizers. Metal complexes in general offer a wealth of excited states that are not available to organic molecules, and [Ru(bpy)3]2+ in particular has been instrumental in fundamental photochemistry and photophysics research. In the case of Ru and Os complexes, intersystem crossing to the 3MLCT state after initial photoexcitation tends to be rapid and quantitative, producing long-lived triplets that can be harnessed for photocytotoxicity, e.g., generating ROS for PDT.
We employ a number of steady-state and time-resolved spectroscopic techniques to unravel the excited state dynamics of our compounds and to represent the sequences as Jablonski diagrams. Luminescent states are characterized by emission spectra and lifetimes, and dark states by differential excited state absorption spectra and transient absorption lifetimes.
Photobiology
Using the underlying photophysics for rational design of photoactive compounds, we are able to install high levels of activity in either an oxygen-dependent (PDT) or independent (PCT) manner. To confirm their photobiological potency for any given application, we screen several human cancer cell lines across a variety of treatments – including variable oxygen tension (0.1–21% O2), multiple light sources and activation wavelengths from high energy blue (453 nm) to low energy near- infrared (733–976 nm), and manipulation of the light dosimetry (fluence, fluence rate, pulsed or fractioned, bandwidth). Through our application-driven and interdisciplinary approach, we have already successfully identified TLD1433, the first Ru-photosensitizer to undergo clinical trials (Phase II, clinical trial identifier NCT03945162), and continue to push the limits in light-activated therapies
 Screening photosensitizers with red (633 nm) and green (523 nm) light
Screening photosensitizers with red (633 nm) and green (523 nm) light