RE:This stock will rocket when we get FDA approvalscockmoses wrote: Just like Tilray. As soon as they hit approval to sell in the US the stock jumped over $100. We will be next with the capsules we are producing in Quebec. We also have CBD vapes that are not allowed here yet but legal in US. Big news will come. I will remind everyone when they do. Cheers
It takes 10-12 years for FDA approval!! Smart investors want to see some sort of results to back up these insane LP valuations. Not going to happen for most since 90% of the LPs hyped huge growing expansions so they could attach a huge forward earnings figure based on square footage!!! If only it was that easy, nevertheless retail ate it up big time, but smart money is saying “show me your business model works first before I invest”. WEED and ACB will take at least 3 years to show any positive earnings. Smart money is not going to play that iffy waiting game while there’s a few LPs that are actually walking their talk.
The top market cap LPs will continue to decline another 40% over the next few weeks and by early 2019 they’re going to crash to 2017 valuations. Unfortunately the top LPs will mostly drag the whole sector down with a few unwarranted ones over the next weeks, but by early 2019 the few that suffered the undeservedly suffered the decline will become the market darlings as the big guys disappoint big time. The bigger they are the harder they fall!
Go ahead and hold to learn the hard way that hype and talk is just that until it’s follwed through. New Drug Approval Process
FDA Approvals
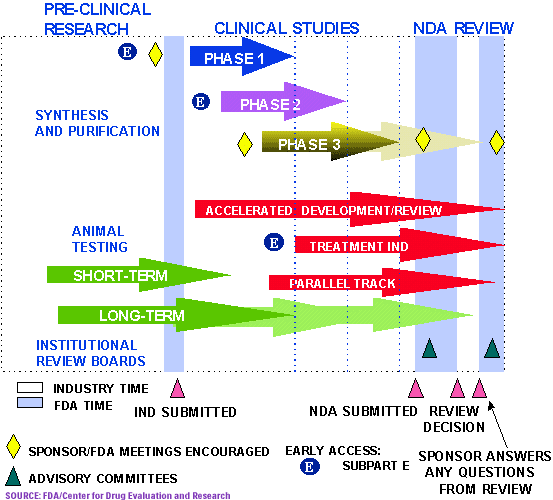
It takes on average 12 years and over US$350 million to get a new drug from the laboratory onto the pharmacy shelf. Once a company develops a drug, it undergoes around three and a half years of laboratory testing, before an application is made to the U.S. Food and Drug Administration (FDA) to begin testing the drug in humans. Only one in 1000 of the compounds that enter laboratory testing will ever make it to human testing.
If the FDA gives the green light, the "investigative" drug will then enter three phases of clinical trials:
- Phase 1 uses 20-80 healthy volunteers to establish a drug's safety and profile. (about 1 year)
- Phase 2 employs 100-300 patient volunteers to assess the drug's effectiveness. (about 2 years)
- Phase 3 involves 1000-3000 patients in clinics and hospitals who are monitored carefully to determine effectiveness and identify adverse reactions. (about 3 years)
The company then submits an application (usually about 100,000 pages) to the FDA for approval, a process that can take up to two and a half years. After final approval, the drug becomes available for physicians to prescribe. At this stage, the drug company will continue to report cases of adverse reactions and other clinical data to the FDA.
The research-based pharmaceutical industry currently invests some US$12.6 billion a year in new drug development. Historically, the drug development figure doubles every five years.
Steps from Test Tube to New Drug Application Review