Encouraging news: three new medical studies show a commonly available anti-malaria drug known as chloroquine aka chloroquine phosphate is showing strong results against COVID-19 infections in both China and South Korea. Excerpts from three studies, including one published in Nature are below.
 quinine word in a dictionary. quinine concept.
quinine word in a dictionary. quinine concept. An Effective Treatment for Coronavirus (COVID-19)
Presented by: James M. Todaro, MD and Gregory J. Rigano, Esq.
In consultation with Stanford University School of Medicine, UAB School of Medicine and National Academy of Sciences researchers.
SPANISH: https://docs.google.com/document/d/e/2PACX-1vR1adodKPhWalV9djnerI2x_v1LGgGyhZZxpl0O5r-ZNyDdagqFq1rTCxXBqaeicfxgvypDOqKCZVyV/pub
Translation by: Celia Martnez-Aceves (Yale B.S. Candidate 2021), Martn Martnez (MIT B.S. 2017)
Summary
Recent guidelines from South Korea and China report that chloroquine is an effective antiviral therapeutic treatment against Coronavirus Disease 2019. Use of chloroquine (tablets) is showing favorable outcomes in humans infected with Coronavirus including faster time to recovery and shorter hospital stay. US CDC research shows that chloroquine also has strong potential as a prophylactic (preventative) measure against coronavirus in the lab, while we wait for a vaccine to be developed. Chloroquine is an inexpensive, globally available drug that has been in widespread human use since 1945 against malaria, autoimmune and various other conditions.

Chloroquine: C18H26ClN3
Background
The U.S. CDC and World Health Organization have not published treatment measures against Coronavirus disease 2019 (“COVID-19”). Medical centers are starting to have issues with traditional protocols. Treatments, and ideally a preventative measure, are needed. South Korea and China have had significantly more exposure and time to analyze diagnostic, treatment and preventative options. The U.S., Europe and the rest of the world can learn from their experience. According to former FDA commissioner, board member of Pfizer and Illumina, Scott Gotlieb MD, the world can learn the most about COVID-19 by paying closest attention to the response of countries that have had significant exposure to COVID-19 before the U.S. and Europe.[1]
As per the U.S. CDC, “Chloroquine (also known as chloroquine phosphate) is an antimalarial medicine… Chloroquine is available in the United States by prescription only… Chloroquine can be prescribed for either prevention or treatment of malaria. Chloroquine can be prescribed to adults and children of all ages. It can also be safely taken by pregnant women and nursing mothers.”[2]
CDC research also shows that “chloroquine can affect virus infection in many ways, and the antiviral effect depends in part on the extent to which the virus utilizes endosomes for entry. Chloroquine has been widely used to treat human diseases, such as malaria, amoebiosis, HIV, and autoimmune diseases, without significant detrimental side effects.”[3]
The treatment guidelines of both South Korea and China against COVID-19 are generally consistent, outlining chloroquine as an effective treatment.
Specifically, according to the Korea Biomedical Review, in February 2020 in South Korea, the COVID-19 Central Clinical Task Force, composed of physicians and experts treating patients agreed upon treatment principles for patients with COVID-19.[4] In China, the General Office of the National Health Commission, General Office of the State Administration of Traditional Chinese Medicine as well as a Multi-Center Collaborative Group of Guangdong Provincial Department of Science and Technology and Guangdong Provincial Health Comp and the China National Center for Biotechnology Development have established effective treatment measures based on human studies.[5]
According to their research (reported in Clinical Trials Arena),
“Data from the drug’s [chloroquine] studies showed ‘certain curative effect’ with ‘fairly good efficacy’ … patients treated with chloroquine demonstrated a better drop in fever, improvement of lung CT images, and required a shorter time to recover compared to parallel groups. The percentage of patients with negative viral nucleic acid tests was also higher with the anti-malarial drug… Chloroquine has so far shown no obvious serious adverse reactions in more than 100 participants in the trials… Chloroquine was selected after several screening rounds of thousands of existing drugs. Chloroquine is undergoing further trials in more than ten hospitals in Beijing, Guangdong province and Hunnan province.”[6]
…
Chloroquine as a prophylactic (preventative) measure against COVID-19[11]
According to research by the US CDC, chloroquine has strong antiviral effects on SARS coronavirus, both prophylactically and therapeutically. SARS coronavirus has significant similarities to COVID-19. Specifically, the CDC research was completed in primate cells using chloroquine’s well known function of elevating endosomal pH. The results show that “We have identified chloroquine as an effective antiviral agent for SARS-CoV in cell culture conditions, as evidenced by its inhibitory effect when the drug was added prior to infection or after the initiation and establishment of infection. The fact that chloroquine exerts an antiviral effect during pre- and post-infection conditions suggest that it is likely to have both prophylactic and therapeutic advantages.”
The study shows that chloroquine is effective in preventing SARS-CoV infection in cell culture if the drug is added to the cells 24 h prior to infection.
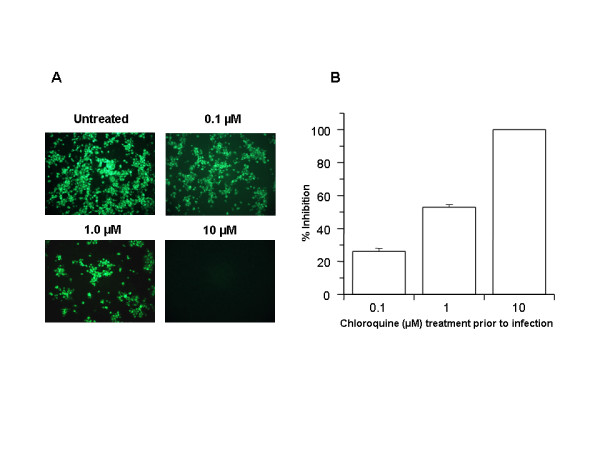
FIGURE 1
Prophylactic effect of chloroquine. Vero E6 cells pre-treated with chloroquine for 20 hrs. Chloroquine-containing media were removed and the cells were washed with phosphate buffered saline before they were infected with SARS-CoV (0.5 multiplicity of infection) for 1 h in the absence of chloroquine. Virus was then removed and the cells were maintained in Opti-MEM (Invitrogen) for 16–18 h in the absence of chloroquine. SARS-CoV antigens were stained with virus-specific HMAF, followed by FITC-conjugated secondary antibodies. (A) The concentration of chloroquine used is indicated on the top of each panel. (B) SARS-CoV antigen-positive cells at three random locations were captured by using a digital camera, the number of antigen-positive cells was determined, and the average inhibition was calculated. Percent inhibition was obtained by considering the untreated control as 0% inhibition. The vertical bars represent the range of SEM.
…
In addition, the study also shows that chloroquine was very effective even when the drug was added 3–5 h after infection, suggesting an antiviral effect even after the establishment of infection.
…
The UK has banned the export of Chloroquine[13]
As of February 26, 2020, the UK government has added chloroquine to the list of medicines that cannot be parallel exported from the UK. Chloroquine was never on this list before. This likely happened because of the growing body of evidence of chloroquine’s effectiveness against coronavirus.
China prioritizes internal use of Active Pharmaceutical Ingredients (APIs) including Chloroquine[14]
In early February, Chongqing Kangle Pharmaceutical was requested by the Ministry of Industry and Information Technology, Consumption Division to promptly increase the manufacturing and production of the active pharmaceutical ingredients chloroquine phosphate despite slowed production during the Chinese New Year.
…
Conclusion
Chloroquine can both both prevent and treat malaria. Chloroquine can prevent and treat coronavirus in primate cells (Figure 1 and Figure 2). According to South Korean and China human treatment guidelines, chloroquine is effective in treating COVID-19. Given chloroquine’s human safety profile and existence, it can be implemented today in the U.S., Europe and the rest of the world. Medical doctors may be reluctant to prescribe chloroquine to treat COVID-19 since it is not FDA approved for this use. The United States of America and other countries should immediately authorize and indemnify medical doctors for prescribing chloroquine to treat COVID-19. We must explore whether chloroquine can safely serve as a preventative measure prior to infection of COVID-19 to stop further spread of this highly contagious virus.
Full study available here