Remember the science? Part 1New paper #1 that mentions TLD1433
14a = TLD1411
14b = TLD1433 Ruthenium Complexes: An Alternative to Platinum Drugs in Colorectal Cancer Treatment Kazi Mustafa Mahmud 1, Mahruba Sultana Niloy 1, Md Salman Shakil 2 3, and Md Asiful Islam 4
1 Department of Biochemistry and Molecular Biology, Jahangirnagar University, Savar, Dhaka 1342, Bangladesh
2 Department of Pharmacology & Toxicology, University of Otago, Dunedin 9016, New Zealand
3 Department of Biochemistry, Primeasia University, Banani, Dhaka 1213, Bangladesh
4 Department of Haematology, School of Medical Sciences, Universiti Sains Malaysia, Kubang Kerian 16150, Malaysia
Received: 23 July 2021 / Revised: 13 August 2021 / Accepted: 16 August 2021 / Published: 19 August 2021
4.14. Photodynamic Therapy
Photodynamic therapy (PDT) has emerged as a potential cancer therapy that is either used as a sole treatment or in combination with chemotherapy, surgery, and/or radiation [179]. PDT uses lights with appropriate wavelength to stimulate photosensitizer (PS) which mediates photochemical reaction to produce ROS and consequently kill tumors (Figure 6) [180].
Ru(II) PSs [Ru(2,2′-bipyridine)2(2-(2′,2″:5″,2′′′-terthiophene)-imidazo[4,5-f][1,10]phenanthroline)]2+ AKA
TLD1411 (14a) and [Ru(4,4′-dimethyl-2,2′-bipyridine)2(2-(2′,2″:5″,2′′′-terthiophene)-imidazo[4,5-f][1,10]phenanthroline)]2+ AKA
TLD1433 (14b) (Figure 7A) contain both photo-biological and photo-physical properties [92].
14b was originally synthesized by Sherri MacFarland and this Ru(II)-based photosensitizer entered clinical trial to treat bladder cancer through PDT. A phase I clinical study was conducted with
14b (at 0.70 mgcm−2 dose) on six non-muscle-invasive bladder cancer patients (NCT03053635) and tumor relapse was not observed up to 180 days [181].
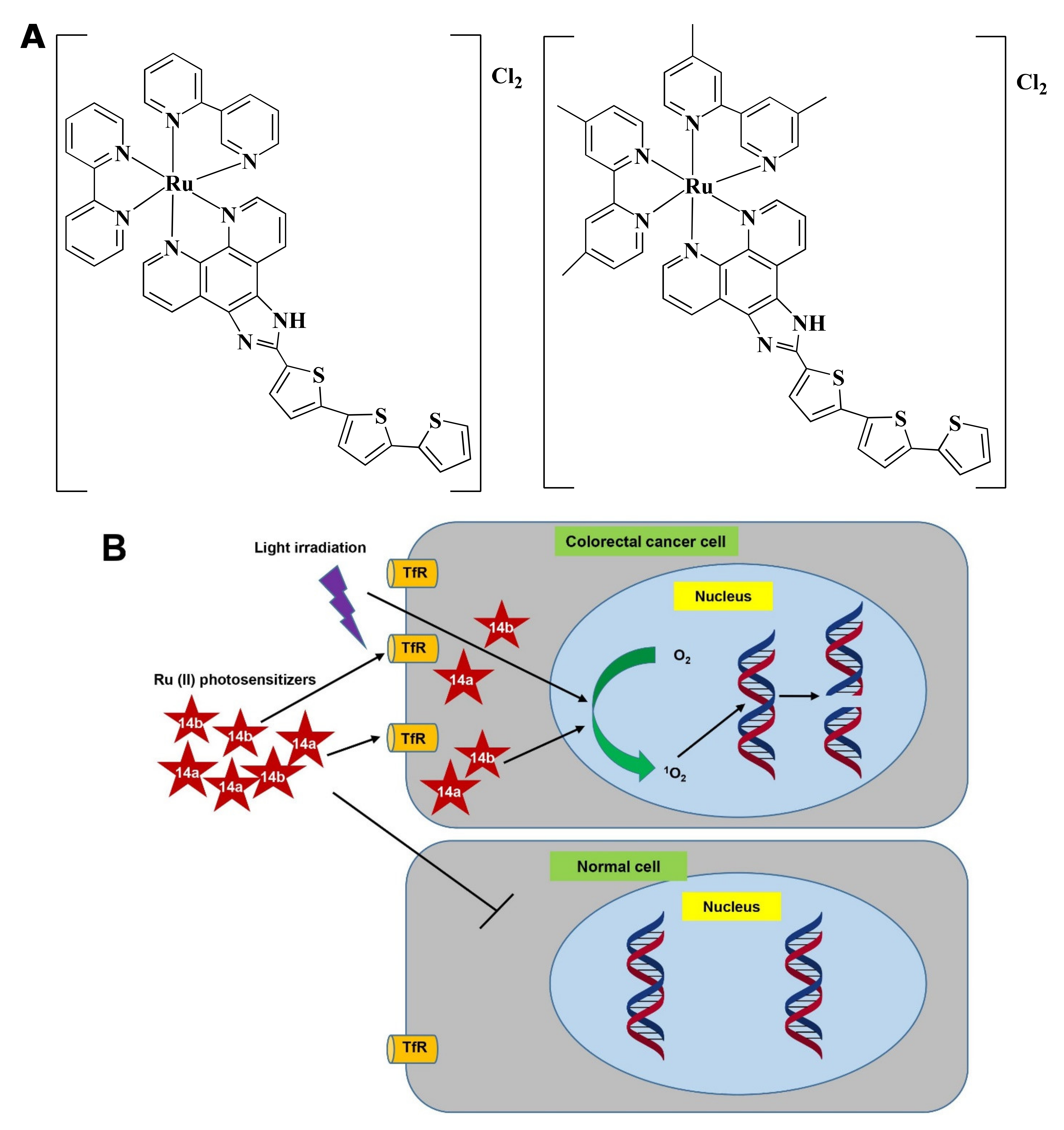 Figure 7
Figure 7. Photodynamic effects of Ru-complex in colorectal cancer and normal cells. (A) Chemical structure of 1
4a and
14b. (B) Ru-complexes having photosensitizing properties selectively kill CRC cells. Ru-complex with photosensitizing property can selectively enter cancer cells, and with the presence of light irradiation, O2 is converted into 1O2. 1O2 interact with DNA to mediate cell death via photocleavage activity. Tryptophan receptor: TfR, Oxygen: O2, Singlet oxygen: 1O2.
Ru(II) in 14a, and 14b makes the complexes specific towards cancer cells rather than normal cells and upon light irradiation increased singlet oxygen (1O2) quantum yield [182]. 14a and 14b induced fragmentation of DNA via photocleavage activities (Figure 7B) [92,183]. According to Fong et al. [92], 14a (4 µM) and 14b (1 µM) exhibited photodynamic effects which cause photon-mediated complete death of CT-26 cells. However, both 14a (10 µM) and 14b (10 µM) showed minimal toxicity (less than 10%) in the dark. The maximum tolerated dose was recorded to 36 and 103 mg/kg for 14a and 14b, respectively. Besides, in vivo treatment of 14a and 14b modulated tumor cell regression. Four hours post-intrathecal administration of 14a (36 and 2 mg/kg), and 14b (53 and 5 mg/kg) in BALB/c mice, followed by irradiation with a continuous wave or pulsed light sources (λ = 525–530 nm, H = 192 Jcm−2) for 30 min (with 30 s on/off cycle) exhibited a higher tumor growth reducing efficacy after 24 h. 14a (2 mg/kg) and 14b (5 mg/kg) delayed the tumor growth for 8 and 9 days, respectively. Both the compounds increased survival time in a dose-dependent manner. However, 14b extended survival time by 5-fold compared to 14a [92]. Treatment with 14b also induced antitumor immunity in the colon cancer-containing mouse model [181]. Considering all observations, 14b could be considered as a promising PDT agent in CRC treatment.
The Pt-based drug, Pt(II) 2,6-dipyrido-4-methyl-benzenechloride (PMB) also induced PDT-mediated DNA damage of CRC cells in a similar way [184]. Since 14b and PMB were not investigated under similar experimental conditions, thus the potency of 14b and PMB could not be compared.