Spanish Research Paper Published Today References TLD1433Dual Emissive Ir(III) Complexes for Photodynamic Therapy and Bioimaging by Marta Redrado 1,Andrea Benedi 2OrcID,Isabel Marzo 2OrcID,M. Concepcin Gimeno 1,*OrcID andVanesa Fernndez-Moreira 1,*OrcID
1 Departamento de Qumica Inorgnica, Instituto de Sntesis Qumica y Catlisis Homognea (ISQCH), CSIC-Universidad de Zaragoza, 50009 Zaragoza, Spain
2 Departamento de Bioqumica y Biologa Celular, Universidad de Zaragoza-CSIC, 50009 Zaragoza, Spain
* Authors to whom correspondence should be addressed.
Received: 28 July 2021 / Revised: 26 August 2021 / Accepted: 29 August 2021 / Published: 1 September 2021
(This article belongs to the Special Issue Metallodrugs for Targeted Cancer Therapy)
1. Introduction
Photodynamic therapy is an underdeveloped cancer treatment that is attracting much attention recently due to the great prospect for destroying tumors and tumor vasculature as well as stimulating the immune response [1,2,3]. This treatment modality is based in three key pillars, a photosensitizer (PS), light and oxygen, rendering toxicity limited to the regions where the three components are together. Traditionally, organic scaffolds such porphyrins, chlorin or bacteriochlorin derivatives are used as PSs relying on their efficiency to generate highly toxic reactive oxygen species (ROS) upon irradiation at a specific light wavelength in presence of molecular oxygen [4]. Ideally, an optimum PSs should compile some common features like: (a) have strong absorption in the red/near infrared (NIR) region of the electromagnetic spectrum to allow the treatment of inner and bigger tumors; (b) have high quantum yield of the triple excited state formation and relatively long lifetime to generate ROS species effectively; (c) have minimum dark toxicity as well as a fast clearance from the body in order to avoid additional side effect to the patients and, last but not less important, (d) have a short and highly yielding synthetic route affording single and well characterized PSs [5]. Lately, transition metal complexes based on Ru, Os and Ir, among others, have been investigated as alternative to commercially available organic PSs to fulfill these premises [6]. In general, transition d6 metal complexes have tunable photophysical properties: high kinetic stability and low photobleaching character. The presence of the metal helps spin–orbit coupling, leading to ultrafast and efficient population of triplet excited states, and promoting high yields of singlet oxygen generation [7]. The successful incorporation in clinical trials of a Ru(II) complex, specifically TLD1433, developed by Prof. McFarland and coworkers for the treatment of bladder cancer with PDT, has encouraged to further investigate in this field, Figure 1 [8]. In addition to Ru(II) species, also Ir(III) complexes have demonstrated their great capacity as PSs [9]. A throughout design leads to Ir(III) complexes exhibiting high quantum yields for the triplet excited state and thus enabling the efficient generation of ROS species [10]. Alternatively, to this therapy function, Ir(III) complexes have also demonstrated to be suitable luminescent probes to target different organelles within the cells. Thus, many of them have been described to selectively localize preferentially in mitochondria [11], nuclei [12] or lysosomes [13] among other inner compartments, or even pass through one organelle to another [14] by simple modification of their ligand scaffolds.
In addition to that, many reports suggest that a PS targeting mitochondria is prone to enhance their potency of photodynamic therapy due to the distinct biological features of mitochondria [15]. In fact, there is a current trend in drug design for the development of mitochondrial targeted drugs due to their indispensable role in the regulation of cell functions [16]. Mitochondria are decisive regulators of apoptosis, and they produce most of the cell’s energy. They contain a high concentration of oxygen, which is one of the three main pillars of PDT. It was demonstrated that even low levels of singlet oxygen produced in the mitochondria are more toxic than large amounts produced in other parts of the cells like cell membrane or nucleus [17]. Actually, many of the commercially available PSs, such as Photofrin [18], Verteporfin [19] or Redaporfin [20,21], partially target mitochondria, where they display the therapeutic potential (Figure 1). In this sense being able to assess the biodistribution of the probe to ensure its successful delivery to mitochondria prior activating the therapeutic function will be key for maximizing its therapeutic potential.
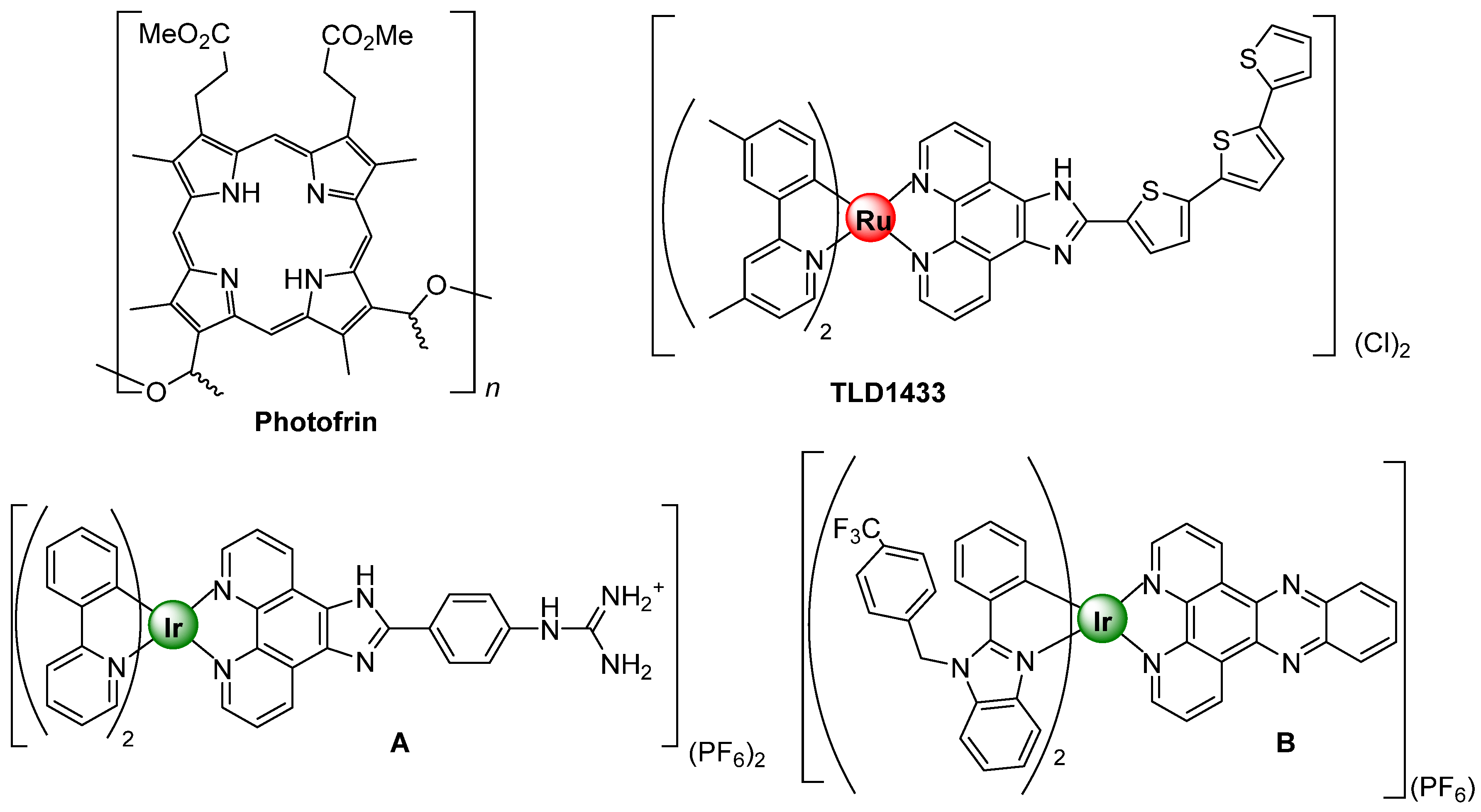 Figure 1. Chemical structures of Photofrin, TLD1433, and complexes (A,B) adapted from [8,18,22,23].
Figure 1. Chemical structures of Photofrin, TLD1433, and complexes (A,B) adapted from [8,18,22,23]. With all this knowledge in mind, we propose to develop a bifunctional mitochondrial selective Ir(III) complex that can act as both, PSs and cell imaging probe, by activating each function with a different irradiation wavelength. The design approach relies on the incorporation of a luminescent tag to an Ir(III) PSs with well differentiated optical properties from that of the metallic fragment, i.e., a spectral photophysical separation should be taken into consideration (Figure 2). For doing so, an organic chromophore would be coupled to any ligand within the Ir(III) coordination sphere. Typically, Ir(III) complexes for PSs rely on the structure of cationic species derived from [Ir(C^N)2(N^N)]+, where C^N represents an orthometallated and N^N a bisimine ligand. Therefore, taking the synthetic versatility that offers the bisimine 2,2′-dipyridylamine (dpa), where a chromophore like anthracene could be easily coupled to the amine as previously described by Zhua [24], we envisioned that it would be feasible the incorporation of analogous chromophores (acridine) using a similar methodology. In this way, an organic chromophore could be incorporated to the Ir(III) metallic scaffold offering the possibility of tracking the complex within the cells, using a different irradiation light wavelength from that of therapy activation. This work is aimed at delivering the first synthetic steps towards a rationale design of bifunctional metalloprobes within the area o PTD and cell imaging agents.