Taking us back to the original thrid party publication in
The Cell when Metablok was proven in animal studies to have potential in treating organ inflammation. Meaning that the same mechanism of action that works in lung also works in the liver and KIDNEY
The Lung 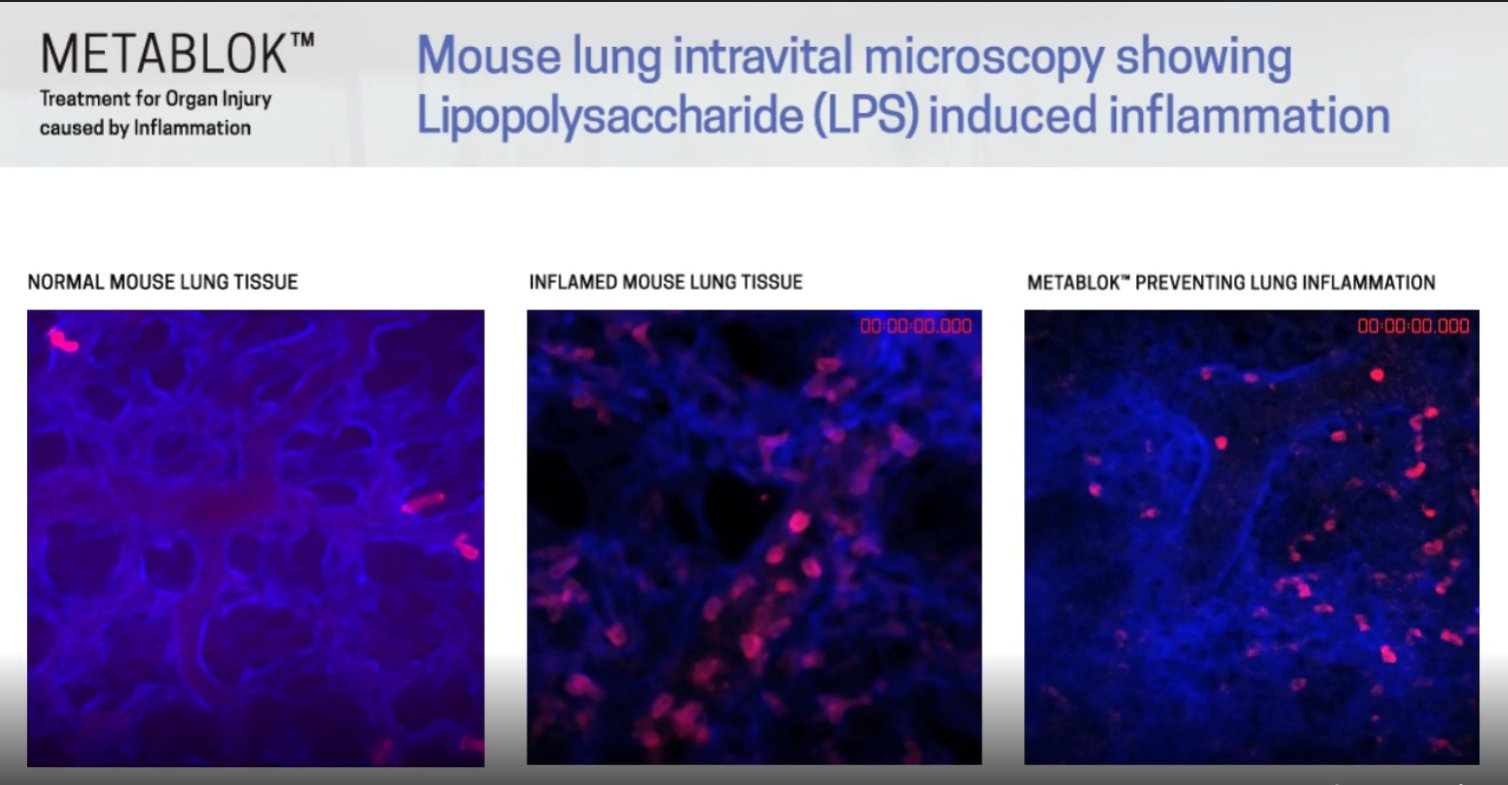 The Liver
The Liver 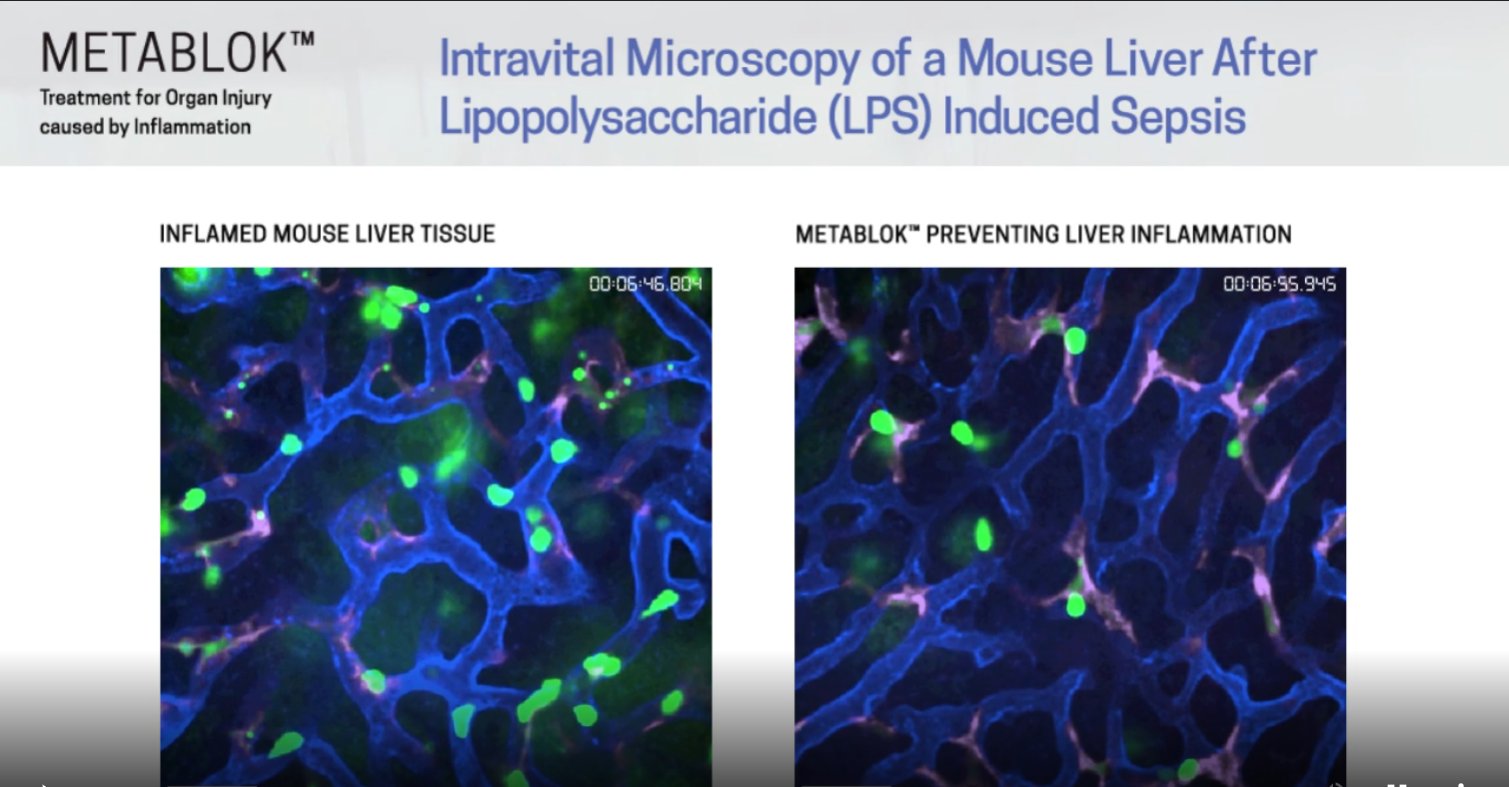 The Kidney
The Kidney 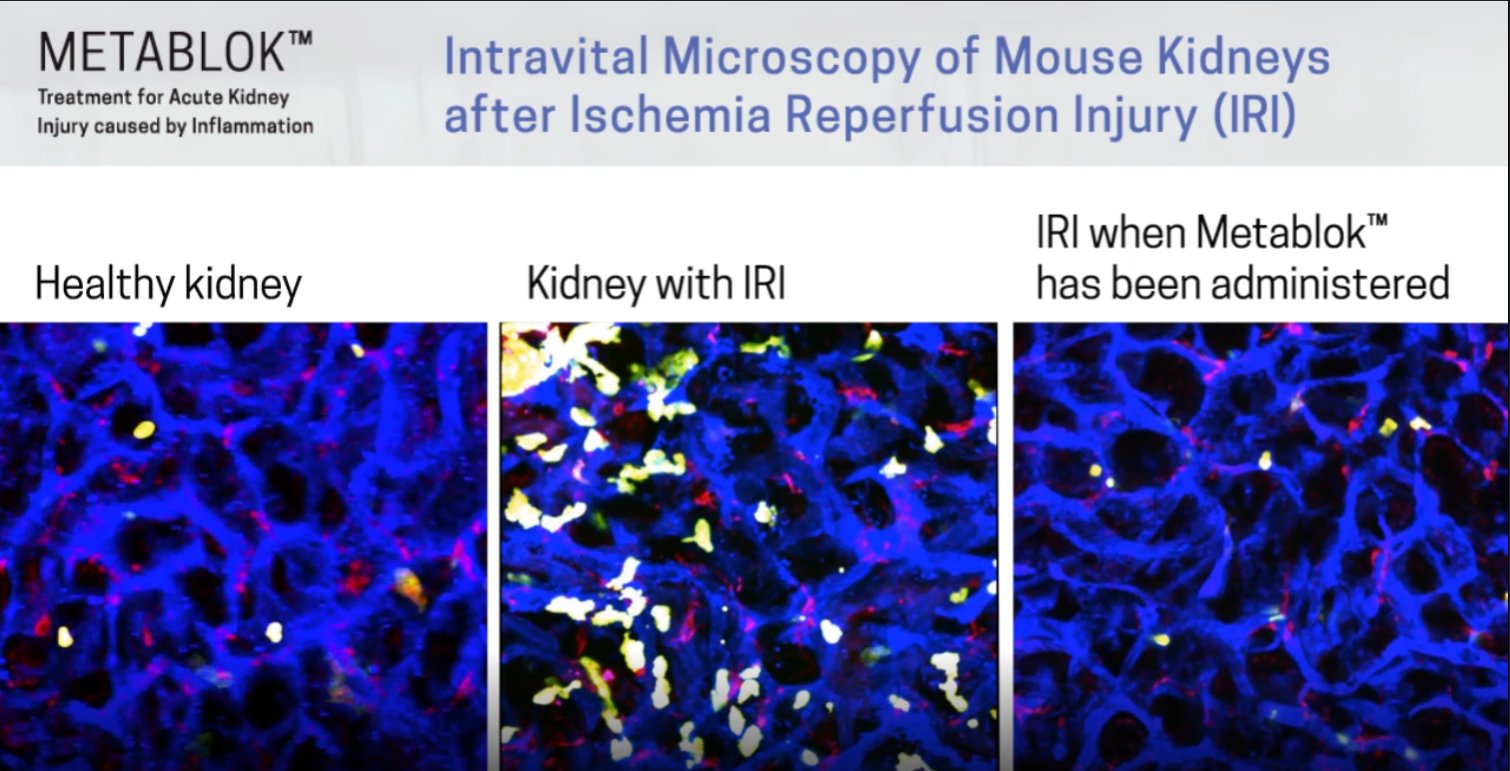
The reason that I have become even more bullish on the potential with Arch Biopartners, is that the original intent was to pursue a Kidney trial as it had the strongest animal data. The company stepped up to the global stage to help in the fight against Covid19 and ran a Lung trial because the animal data provided the opportunity. Given now that we know the technology is real with the release of the Phase II data, this novel therapeutic for organ inflammation can impact the market at a much larger scale. If the lung was validated, then in theory it drammatically derisks the Liver and Kidney.
- Currently, no specific therapies exist to prevent AKI in the world today. Management of AKI is supportive and includes life-sustaining therapy with dialysis. Patients that experience AKI are at high risk of developing chronic kidney disease, adverse cardiovascular outcomes and death.The worldwide market for AKI is estimated to be over $20 billion USD per year. ($325.4 dollars a share)
- CEO of Arch Biopartners, Richard Muruve, commented, “With the license of Telara’s cilastatin patent rights combined with our own, we look forward to doing a significant Phase II trial targeting ischemia reperfusion related AKI. Currently, there are no effective treatments to prevent AKI and Arch now has both Metablok and cilastatin that can be used in separate patient arms of a new Phase II trial. The collaboration with Telara will also facilitate a future drug application in Europe and potential patient recruitment in Spain.”
The potential this novel therapeutic has as a one stop shop in organ inflammation is incredible and I look forward to the upcoming trial to continue to build shareholder wealth.
I am a shareholder, this is not investing advice and contains opinions, Do your own Due Diligence, I have provided mine in a previous post.