CancerSlayer wrote:
winr88 wrote: I definitely agree that very positive results for the 180 day and 90 day trials will be reported. I have mixed feelings however how much the share price will increase despite kick A$$ CR results.
I wonder how many in the market are thinking “too good to be true” and are waiting for the other shoe to drop regarding negative health effects or a big correction in trial results to the downside. It doesn’t appear to me any of those negatives will occur. The share price should move substantially upward. We will soon see.
Although I consider myself an educated & objective man, a little superstitious sentiment creeps in from time to time. I'd hate to see comments re: predictions made by a negative market jinx our results...we can leave that to the sports announcers ; ). I actually think very few are thinking this tech is "too good to be true". On the contrary, many think that certain past "perceived" negative events (I.e. protocol changes, treatment-unrelated death, mgmt. changes, limited tech-biosimilars under clinical study, etc.) are predictive of outcome. But we know better...
Speaking of kick A$$ CR results, I am hoping we have continuing & equally important kick A$$ safety results. I'd give up a few percentage points in CR for guaranteed favorable safety results.
Meanwhile, it's always important to keep your eye on the competition....came across the following, which is the most recent look at what may end up being our strongest competition (N-803 + BCG for CIS disease):
Among Cohort A (CIS), there were 83 patients enrolled. With a 23.9 month median follow-up, the complete response rate was 71% (95% CI 60.1%, 80.5%), with median duration for 3-month responders of 24.1 months and a 55% probability of maintaining this complete response for ≥ 18-months (95% CI 40.1%, 67.3%). The cystectomy free rate in responders was 93%, with a 100% cancer specific survival at 24-months. The 12 month (62%) and 24 month (52%) durable complete response in Cohort A is as follows:
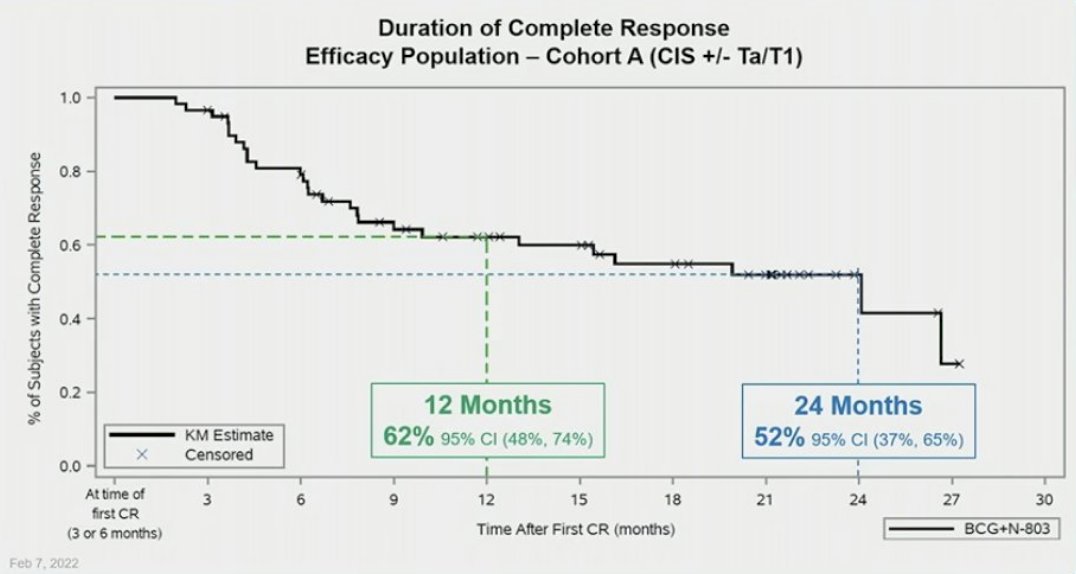
Despite the promising results above, this is a treatment-intensive option that requires BCG (in short supply for the foreseeable future) & that not every patient can/will comply with. The FDA, medical community & public are demanding more than one option for this deleterious disease that is also one of the most expensive cancers to treat. There's certainly room for TLT to make its mark. The data will speak & the only other shoe I'm waiting to drop is TLT's success. JMO. Good luck...