$TLTFF Study II Preliminary Results As of November 29, 2021 - Study II Preliminary Results
As of November 29, 2021, Study II has enrolled and provided the primary study treatment for 30 patients (including three patients from the Study treated at the Therapeutic Dose) for a total of 33 patients, demonstrating the following interim results:
Note: Significant clinical data is still pending in Study II and drawing conclusions from this interim clinical data set and assumptions should be done with caution, as Study II is still ongoing and new clinical data collected may or may not continue to support the current trend.
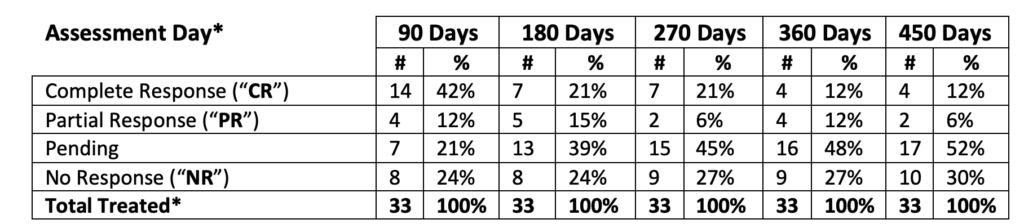
*Includes three (3) patients treated at the Therapeutic Dose form Phase Ib NMIBC Clinical Study (2-CR and 1- NR at 90, 180, 270, 360, 450 days)
An analysis of the Study II clinical data (with 3 patients from Study Ib) provides the following interim assessments:
- 7/10 patients (70.0%), who achieved a CR at 90 days continue to demonstrate CR at 180 days
- In the total population of 33 patients (@ 90 days):
- 42.4% achieved Complete Response (“CR”)
- 12.1% achieved Partial Response (“PR”)
- 21.2% are Pending
- 24.2% achieved No Response (“NR”)
Hence, the potential for CR is up to 75.8%** for the interim clinical data analysis.
Note**: Assumes both PR and Pending data are clinically determined to be CR at a later assessment date.
- In the total population of 18 patients (@ 90 days), who received the optimized treatment:
- 44.4% achieved CR
- 11.1% achieved PR
- 38.9% are Pending
- 5.6% achieved NR
Hence, the potential for CR is up to 94.4%***
Note***: Assumes both PR and Pending data are clinically determined to be CR at a later assessment date for the interim clinical data analysis.
In summary, for patients who received the primary optimized Study II Treatment versus the original Study II Treatment (90 days), there is a 5% increase in CR and a 77% decrease in NR