SODIUM PEROXIDE BIG SUBJECT -
Using " sodium peroxide " to detect - nickel in ores complex
ores makes for a great - argument. ( debate )
ONLINE EQUATION -
NiCl2 + NaOH + H2O2 = NaCl + Ni(OH)3 Sodium Hydroxide + Hydrogen peroxide = sodium peroxide
Silicate nickel = Nickel chlooride
Mixed together
= Sodium chloride
= Nickel hydroxide
WHAT'S THE SIZE OF THE NICKEL HYDROXIDE ?
*** newly created - large compound structure
One has just increased the molecule structure exponetially
https://pubchem.ncbi.nlm.nih.gov/image/imgsrv.fcgi?cid=61552&t=l 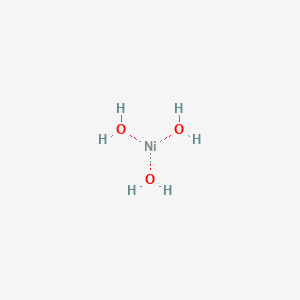
CONVERSION + SIZE IS IMPORTANT -
ICP
metal analysis can be performed on solid and liquid samples, but a solid sample
must be converted to liquid form before testing by dissolving the sample in a solvent
(typically acid) to produce a solution.
The sample solution is introduced into the ICP as a fine aerosol of droplets. The aerosol is produced by a nebulizer which aspirates the sample with high velocity argon to form a fine mist.
The aerosol then passes into a spray chamber where larger droplets are removed. Droplets small enough to be vaporized in the plasma torch pass into the torch body, where the aerosol is mixed with more argon gas. ICP stands for Inductively Coupled Plasma, which is an excitation source generated by directing the energy of a radio frequency generator into a suitable gas. A coupling coil is used to transmit radio frequency to the heated argon gas, producing an argon plasma located in the torch. The hot plasma dries any remaining solvent and causes sample atomization.
The ICP-AES spectrometer detects the atomic emissions produced as light. With ICP Mass Spectrometry, the process uses ionization. The resulting mass of the ions indicates the elements present in the sample.
ICP LAB ANALYSIS - LINK - https://labtesting.com/services/materials-testing/chemical-analysis/icp-analysis/ MOLECULE SIZE Nickel oxide can have a single bond of one oxygen,
or... multiple nickel values with multiple oxygen.
More nickel per (
area vollume )
A nickel hydroxide has one nickel with several
outter branches of - hydrogen and oxygen
creating more area volume but with less nickel.
WHAT DO I THINK ? Juniors like ( cnc ) who only used straight peroxide
were smart - they created a vantage of nickel conversion
in a concentrated value with a straight peroxide.
VERSUS - A junior using sodium peroxide ONLY ONE NICKEL
molecule is attached to several - oxygen and hydrogen.
THUS creating more area volume.
WHICH....in my opinion, can be bothersome.... in the context of,
if ICP testing - uses
just a droplette of solution the amount of nickel with in - a newly created complex bond
having more area mass and volume and only one nickel
could result in -
lessor nickel value detection. Verus - other oxidants that condence nickel into a molecule
that has 2 or more bonds.Thus, increasing the concentration
which ultimately increases - percentage per tonne.
Hey.... were talking, small droplette of water - misted into a
plasma spectrometry machine which has to detect amount of metal,
and some do say.. .ions - with in a mist droplette.
I would think... expanding a nickel bond into a compound bond
would result in - less nickel detected due to a larger molecule
made by - specific oxidants.
I also think... the ICP MS version - which detects, ions,
could be far better - detecting both salt nickel + metallic nickel.
Take for example - Nickel sulphide + plain hydrogen peroxide H202 - like cnc used.
only converts the minerals to oxides or hydroxides
huge advantage with - multi nickel bonds.
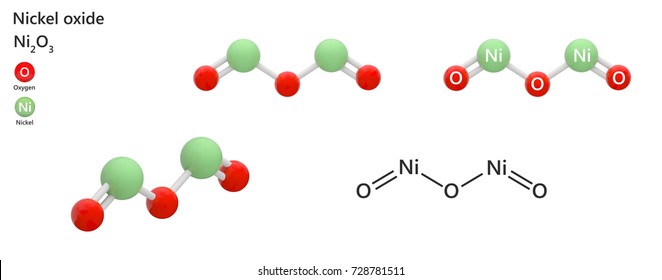
Here is a nickel sulphide converted to nickel oxide.
It has a double nickel bond....
Now... think small droplette of water
and how many more nickel bonds could fit into
a small droplette versus - nickle hydroxde
open link to view - double nickel bonded to 3 oxygen.
now compare a nickel hydorxide molecule above,
i'd say the nickel hydroxide would have less nickel
due to a far larger bond volume area - thus, less nickel
detection.
Historical percentages around the
Langmuir - 1 + #2 Mines
were a good doubling of present values.
former juniors used diff - acids.
Just saying.
Something to think about.
Perhaps our junior should do a double take ?
Hey... i know i would.
lol
https://image.shutterstock.com/image-illustration/nickel-oxide-inorganic-compound-formula-260nw-728781511.jpg
What am i thinking ? - lol
i think the labs should compensate for a - new compound they made
compare to historical ( acids - sulphur + hcl ) where values were
far higher in percentages - and - configure a parameter that adjusts for
the new area volume created by the new compound - and know that
if it were a simple compound - more nickel would be present per -
vapor mist -
nickel sulphide has far less ( compounds ) and more nickel
sulphide has 2 nickel bonds
nicke loxide has- 2 nickel 3 oxygen
nickel chloride has - 1 nickel 2 chloride
nickel sulphide has - 2+ nickel 2 sulphide
Again.... what does a hydroxde nickle look like ?
Yup.... that's alot of area volume -
makes one wonder what a mist of vapor would hold
if nickel was converted to - hydroxide...

And of course.... throwing in a - sodium with hydrogen peroxide
could create a competing - whereas, sodium needs to keep a bond
and takes up more ( area volume ) in final solution being tested.
less space for - nickel - per mist vapor.
If i've errored... by all means....
chime in and correct.
I just couldn't help but notice....
higher grades in the former reports vs new oxidant
was used that was indifferent than former...
Cheers....