CALCIUM vs VANADINITE I' ... VANA Deposit = VANA - dinite ( tease )
Vanadinite is a mineral belonging to the apatite group of phosphates, with the chemical formula Pb5(VO4)3Cl.
Pb is a defunct uranium
V is a phosphate
Cl chlorine is a uranium mobilzer
Water is a uranium mobilzer
Calcium is a uranium mobilzer
Let's suppose,
Ivana's phosphates ( phosphate, potassium ) harbor the uranium.
Vana dinite is nerely a tag along in matrix.
Same with chloride.
Water ( oxide and hydrogen ) and atmospheric air is needed to spark reaction - transition.
Rain water, river waters create movement assisting in errosion.
ACIDS ( base, alkai )
phosphoric
potassic
chloride
sulphide ( iron, pyrites )
calcium lime
= assist solubilty - creating mobility in water
Would seeking vanadinite Pb a good pathfinder, how about lead ?
I would say NO.
It would limit the junior to just vanadinite uranium vs various species of uranium.
There are far more uranium species than.... judt vanadinite.
Carnotite is a potassium uranium vanadate radioactive mineral with chemical formula K2(UO2)2(VO4)2·3H2O
The water content can vary and small amounts of calcium, barium, magnesium, iron, and sodium are often present.
Remember vanadinite has the chloride.
Surrounding geology has calcium
Potassic acids from potassium
Add water ---------> uranium will go mobile.
It will fraction.
Uranium has a strong affinity to bond to... Calcium
Chloride - Calcium - Iron - Magnesium - Manganese -------> ( especially calcium )
Occurrence and Mobilization of Uranium in
Groundwater in Nova Scotia
superb read
https://novascotia.ca/natr/meb/data/pubs/cs/cs_me_2013-001.pdf
What did bsk find top end of Picos Salar - central zone ?
Calcrete - Calcium uranium
And mentioned mobilty
Scotia report above spesks about uranium in well water and Calcium
The calcium created 2 kinds of uranium bonds.
One of the calcium uranium bonds has a - zero valance.
When an atom has eight electrons in its outermost shell it does not share or gain or lose any electrons i.e. its valency is zero. Such atoms having valency zero are known as zerovalent.
Jul 3, 2022
I'll assume calcium / uranium creates --------> stable valance bond with uranium..
Tames, stabilizes.
Could calcium bonding to uranium
alter uranium's radioactive cycle ?
Could calcium be uranium's equalizer ?
Could calcrete uranium be in a dormant phase - low radioactive readings ?
Would removing calcium carbonate / any carbonate from an uranium bond
remove a calcium inhibitor - reactivating the super fast cycling of, alpha, beta, gamma decay ?
There is next to zero resesrch on this subject.
( that i can find online )
ODDITY ?
I have tried several sesrches to find a Uranium calcium bond - zip !
lol
Remember
Potassium 40 ( radioactive ) turns to calcium
And there exists a Calcium 40 - ( radioactive )
Uranium is always present in, Potassium, Phosphate
Calcium, Calcium carbonate,
Clays -------> which contains calcium
Clays absorb uranium.
Form bonds ( zero valance bonds )
Kind of reads as.... calcium is a buffer opposite of uranium.
natures stabilizer.
I'd like to see bsk revisit central zone.
collect a few calcrete uranium low reading samples.
perform a cation exchange with chloride to transfer the uranium to chloride
and take a new reading of the uranium chloride.
If decay radiation increases
it would mean calcium carbonate acted the zero valance full 8 valance of Ca
outter orbital constrainer. of decay cycling.
Proton Nuetron unstable structure causing the accellerated radiation emmission.
Calcium could be the defence bloccker - or - cycle speed reducer.
Picture an atom
then bonded to a calcium carbonate
calcium carbonate would more the uranium
8 calcium electrons ( either +/- )
wrapping the uranium with outlier valance
And why is it they call this effect a - zero valance ?
nuetral effect ?
weak calcrete uranium
could be a great parhfinder to finding dormant uranium ?
Potassium metal, very reactive with water
Calcium reacts vigorously with water, liberating hydrogen forms calcium hydroxide, Ca(OH)2
There are two most common and stable valence states forms of uranium in nuclear waste: the most oxidized U(VI) occurs as the highly soluble and poisonous uranyl species (UO22+), while the reduced U(IV) phase is less toxic and poorly soluble under anoxic conditions
(Choudhary and Sar, 2015, Kumar et al., 2021).
https://www.sciencedirect.com/science/article/abs/pii/S030438942201812X
reduced U(IV)
poorly soluble
= calcium carbonate is poorly soluble
= does calcium cause the reducing ?
U235 is an isotope of uranium.
While UO2 is uranium dioxide, and contains U235 and U238.
Uranium will fraction ( mobilize in water )
Uranium loves to bond to calcium
Salar contains silts / clay / calcium acts as uranium trap
I read a report last eve ------------> speaking about elevated uranium in LAKE silts.
Silts offered up 4 ppm U
4 pom = 1 liter
= 4000 ppm
= 0.4% uranium ( ivana avg carnotire is - 0.039% ) extra decimal 10x less
Yet.... if we anslyze the 2 m downhole lense
white calcrete = calcium = 0.034%
sand gravel gypsum = calcium 0.158%
2.74% grades red rock or more like green sludge = 2.74%
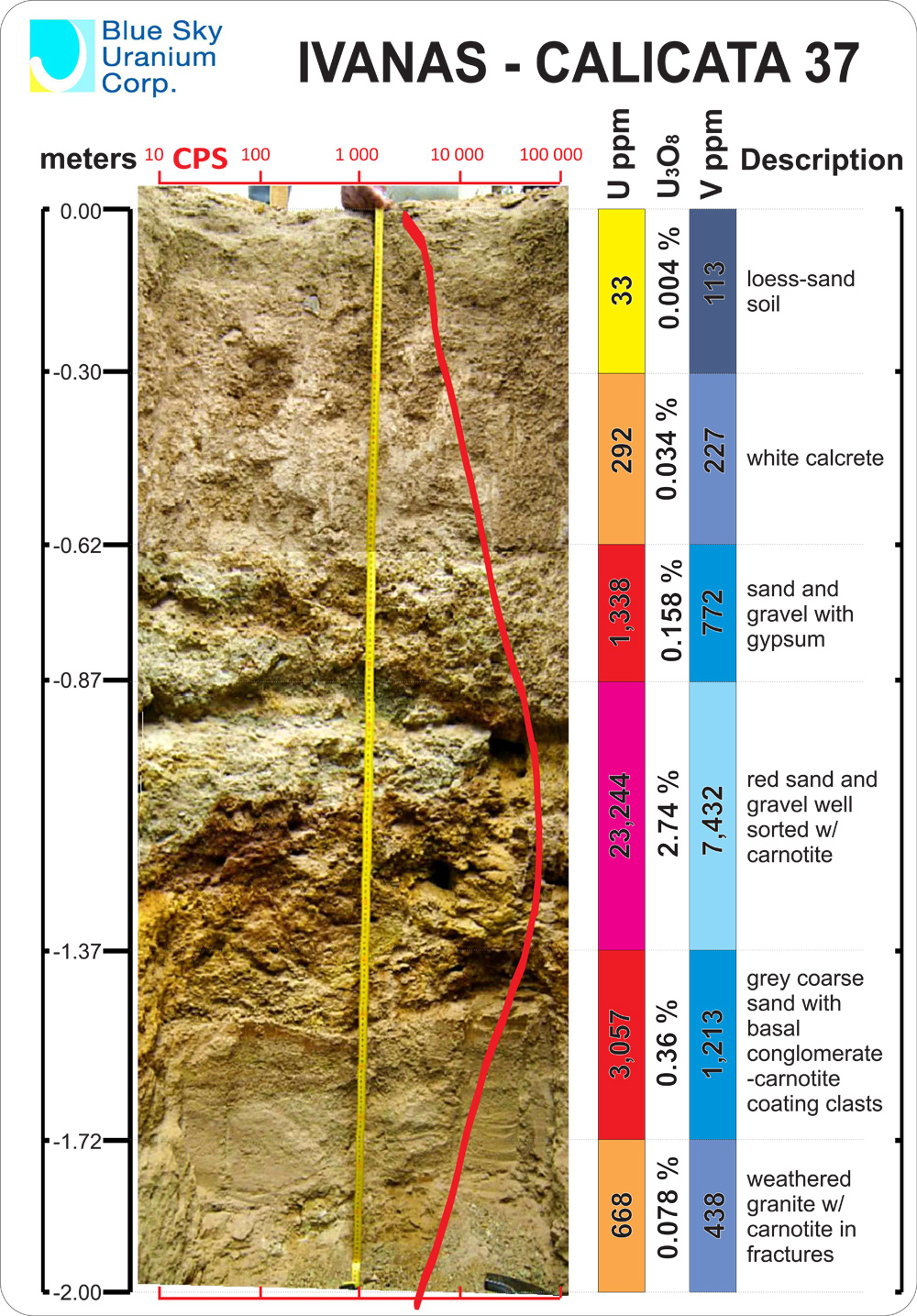
Since calcium forms very strong bonds to uranium.
sedimrnt clay act as a sink trap - trapping uranium
but... calcium forms zero valance
calcrete uranium ( central zone ) revesled mobilty / wesk grades
mendoza lab switch up - was this at height of centeal zone testing - contested grades ?
= should calcium be revisted as a key uranium vector ?
calcium
acts as a fractioner ( with water ) mobilzes
acts the collector of uranium
acts as zero valance
acts the parts of uranium concentrator in silt beds
acts the part of Ph to assist cation, conversion
acts the part of powdering ( with help of air, oxygen )
calcium could be the preferrential go to -------> to anass lots of uranium.
Salar silt beds.
URANIUM is in the purple green rocks
The trick is letting nature concentrate the uranium.
Calcium collects and concentrates ----------> plays that role perfectly.
Westerly of Ivana
Massive open flats
Are several small salars 1 km and under length x 500 m under wide
Seen on satellite
dark purple rocks surround
Each of the small salars have bluffs - purple rocks - rain errosion entering salars
Several have colorstions of, dark green, light green and yellow waters.
One sm salars edges host distinct yellow caking / sands.
Argrntinas Salars
are the concentrators.
Period.
Former post correction
sad part.... most of the resesrch places focus on the porus sands - weak grades....lol
A - typical of uranium sector - tease.
Salar sediments is where i'd place most focus.
Nature already performed the concentrating.
If surround bluff harbor low grade uranium in phospates and potassium
high odds salars have sediment silts with calcium carbonate beds - uranium trapped.
Cheers....
Lots more could be discussed on the subject of - uranium.
i've tried best to condense what i felt is most importsnt.
Yes, wordy.
But uranium is perhaps -------> one of those subjects that is never ending.
There is an enormous amount of resesrch performed on uranium.
I'm trying to fill the gaps where info is a tad lean.
The good stuff ( info ) i seek is just not spoken of nor offered in resesrch.
Whether privlaged info or... no resesrch has yet been performed.
At leadt i've entertained shareholders during this lack of news cycle.
Wink.