“Combining the diagnostic—EAA—with the intervention—PMX—addresses the root cause of the problem and changes the equation for patients with ESS,” Dr. Kellum suggests. “Guided by EAA, Spectral’s unique therapeutic hemoperfusion device, PMX, is highly effective in removing the sepsis-causing endotoxin from the bloodstream using the electrochemical properties of Polymyxin-B, while avoiding side effects of its systemic administration. The device selectively absorbs circulating endotoxin, rebalancing the immune system leading to a decrease in inflammatory mediator levels and improvement in vascular function and hemodynamics. This device and therapy are poised to become the de facto standard of care for ESS,” he adds. 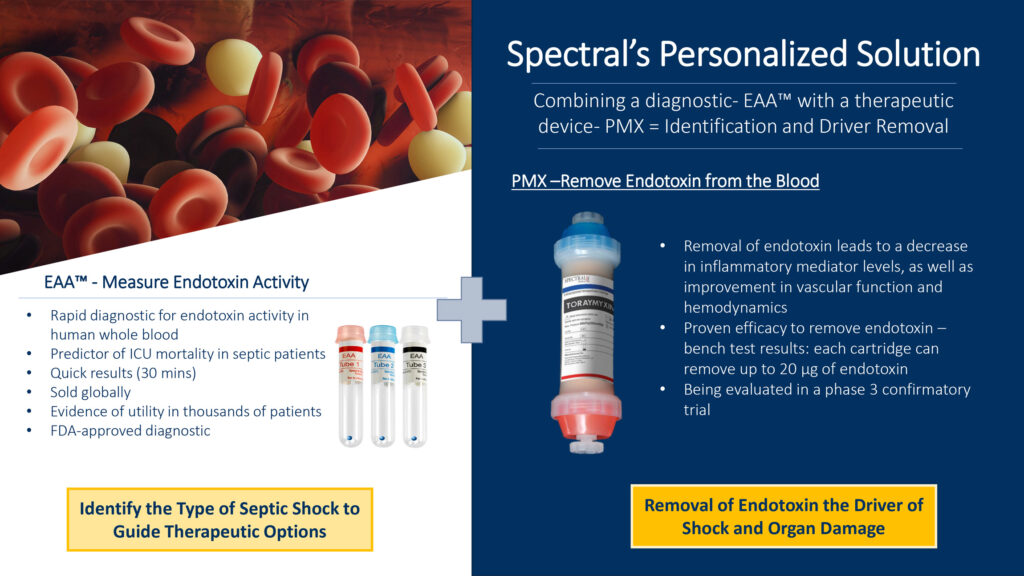
In 2022, PMX received FDA Breakthrough Device Designation for the treatment of endotoxic septic shock. PMX, available in Canada, where Spectral holds exclusive rights, and is approved for therapeutic use in Japan and Europe, having safely and effectively treated patients more than 340,000 times to date. As of 2019, more than 200 scientific papers and peer-reviewed articles have been published concerning PMX and large databases from Japan show highly significant results.
Clinically available since 1995, Spectral secured exclusive U.S. developmental and commercial rights for PMX in 2009, followed by an exclusive Canadian distribution agreement in 2010. Both EAA and PMX are licensed and available commercially in Canada by Health Canada.
Currently, the company is enrolling patients for its Tigris trial, a Phase 3 confirmatory study designed as a 2:1 randomized trial of 150 patients to evaluate the use of PMX in addition to standard care versus standard care alone in adults treated for endotoxemia and septic shock. “We continue to enjoy very strong activity at our sites and we remain confident in finalizing full Tigris enrollment around year end 2024,” Dr. Kellum says.
Tigris is being conducted at 22 sites throughout the U.S. and is a follow-on trial to the company’s initial EUPHRATES trial, which showed benefit but not statistically significant enough for FDA approval, Mr. Seto says. “However, since the initial trial was robust, and this is a huge unmet need, we analyzed our data further and found that out of a subgroup of 179 patients with certain parameters around organ dysfunction and endotoxic activity level ranges between .6 and .9, there was an approximately 10% absolute mortality benefit. With this evidence, we embarked on the Tigris trial.”
Mr. Seto adds that the Tigris trial design is unique in that the company will build on knowledge gained from the EUPHRATES subgroup of 179 patients and combine it, through Bayesian analysis, with data from the 150-patient confirmatory trial, as part of its FDA regulatory submission.
In terms of commercialization, Mr. Seto informs that in 2020, Spectral entered into an exclusive distribution agreement with Baxter (NYSE: BAX). “There is no better partner for our EAA/PMX device and therapy than Baxter—our device actually runs on their CRRT machine,” he says. “They have a large multi-disciplinary team dedicated to the commercialization launch of our device, and Baxter has a greater than 50% share of the CRRT market in the U.S., making this a natural partnership for us.”
Looking ahead, Mr. Seto emphasizes that Spectral’s 30-year track record of clinical and regulatory excellence lends the company significant confidence in navigating the regulatory pathway for its device and therapy.
“We are highly differentiated and competitive in the ESS space, which currently has no other therapy beyond standard of care, and a U.S. addressable market of more than $2.1 billion annually,” he adds.