Amarillo is said to have abundant Coffinite.
Coffinite is a known silicate uranium species mined elsewhere around the world.
Yet... if we delve into Amarillo's Coffinite there are a few hints and clues that might
provide a few more answers.
- labelled coffinite but - migt not be.
- emits ( - beta )
- silicate formula but seen as, brown, black (
big clue )
- coffinite sits closely to the parent uranium forms as....
oxide - pyrite (
irons ) in geology report
Amarillo Geology https://miningdataonline.com/property/4547/Amarillo-Grande-Project.aspx What does Iron do to Uranium ? ( aqueous phase ) by C Noubactep · 2006 · Cited by 180 — The effectiveness of elemental iron (Fe0) to remove uranium (U) from the aqueous phase has been demonstrated. Ahh, iron acts as a sorbent.
Can't help but think... Picos green sludgy silts loaded with uranium in aqueous phase
meets iron ( tail end of picos )
iron bonds to uranium
= brown, black oxide forms as it dries subgrade
Detectable at lab if in iron bond ? Iron also hides the uranium in bond.
What does calcium do . ( calcium is present in Ivana ) Calcrete
Here's a research paper speaking on, calcium and iron.
Notice
U6+ species These
spectral features were undetectable in carbonate- or oxalate-leached solids, suggesting solid phase and sorbed U(VI)O
22+ species are extracted by the leach solutions. Uranium L
3-edge x-ray absorption spectroscopic (XAFS) analyses of the unleached U-Fe oxide solids with less than 1 mol% U reveal that U(VI) exists with four O atoms at radial distances of 2.19 and 2.36 and second shell Fe at a radial distance at 3.19 .
Because of the large ionic radius of UO
22+ (∼1.8 ) relative to that of Fe
3+ (0.65 ), the UO
22+ ion is unlikely to be incorporated in the place of Fe in Fe(III)-oxide structures. Solid-phase U(VI) can exist as the uranyl [U(VI)O
22+] species with two axial U-O double bonds and four or more equatorial U-O bonds or as the uranate species (such as γ-UO3) without axial U-O bonds. Our findings indicate U
6+ (with ionic radii of 0.72 to 0.8 , depending on the coordination environment) is incorporated in the Fe oxides as uranate (without axial O atoms) until a point of saturation is reached. Beyond this excess in U concentration, precipitating U(VI) forms discrete crystalline uranyl phases that resemble the uranyl oxide hydrate schoepite [UO
2(OH)
2·2H
2O]. Molecular modeling studies reveal that
U6+ species could bond with O atoms from distorted Fe octahedra in the hematite structure with an environment that is consistent with the results of the XAFS. The results provide compelling evidence of U incorporation within the hematite structure. Link
https://www.sciencedirect.com/science/article/abs/pii/S0016703702009535
As mention in former posts Focusing on unaffected hardrock and bluffs = allows better identification of U speciations.
In the valley + water = will keep a geologist spinning along with lab trying to figure out
what type of uranium they have with numerous bond - advents due to morphing.
Isotope separation
Parent from subsets
Could explain the Beta - coffinite.
What if it were a beta isotope separated from parent 238 bonded to iron / calcium ?
= beta - ( high speed electrons ) useful.
Water would be that catalyst in which facilitates numerous other U bonds
Roll Front U deposits speak about parent uranium separating from sub sets
Uranium ore transitioning whereas part of the U lags behind
sub set advances ahead in head front geology.
= Disequaliberium. Reuniting each ore body Uranium finds
= Equalibrium Field testing can be inaccurate.
Low readings may in fact be far higher and visa versa.
Uranium deposits at tail of Salar will most likely host a variety of - uranium species.
In and out of equalibrium and several morph / oxidized phases withh other elemental bonds.
Great Read - CIM Uranium ( speaks of roll fronts - equalibrium - disequalibrium )
Gamma reading may be richer or lessor.
Gamma is not the target rather - U235 = Alpha
https://www.cim.org/media/6798/industry-comment-draft-cim-uranium-leading-practice-guidelines.pdf?utm_medium=What%27s+trending&utm_source=CIM+home+page&utm_campaign=Jan24 Could Picos Salar / Laguna act as a water body roll front ? Water oxidizing uranium ( caralyst ) separating uranium
leaving behind some / most U235 in Picos was and green sludgy silts ?
Front roll separated sub U sets ?
Might also apply to upper Picos
Calcrete uranium
Exceprt above points out
These spectral features were undetectable in carbonate- or oxalate-leached solids, suggesting solid phase and sorbed U(VI)O22+ species are extracted by the leach solutions.
Water, Calcium and Iron have sorbent affects on uranium.
Water = U6+ uranyls moblie
Calcium = Clay silts
Iron or other mineral - water facilitates other bonds ( Ca, Mn, Fe act as sorbrnt collectors of U ) I've also came across research on.... Tungsten and Uranium bonds.
San Martin = Tungsten mine aside of Ivana.
Highest U grades are seen in ?
Gravels with no basement ( in process of reforming stone secondary formation )
Green sludgey silts ( water affect ) brought in from Picos ?
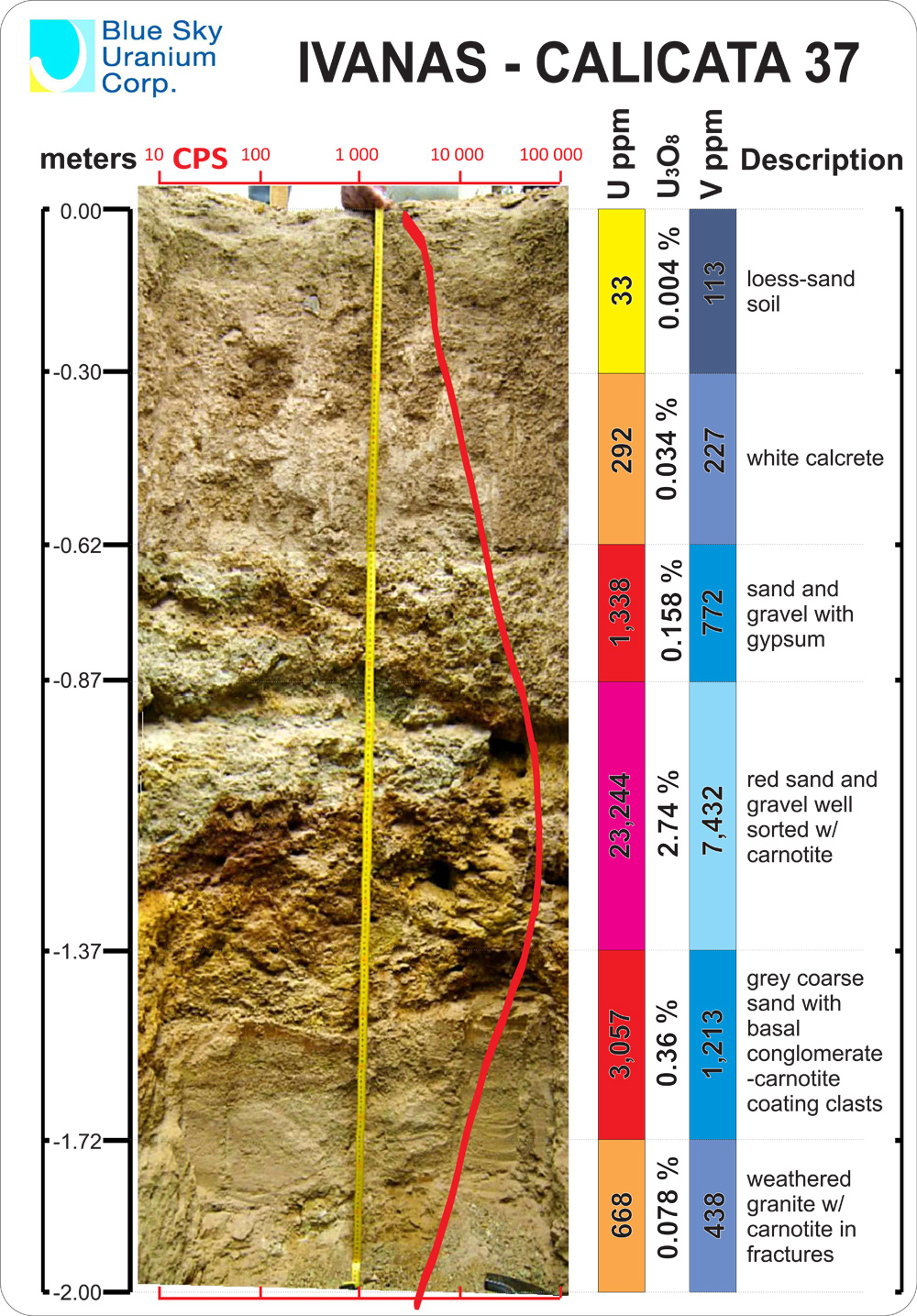
Tad more info i thought i'd share...
Iron bonded with uranium ( brown, black oxide ) or... coffinite ?
Uranium hides inside of iron.
...
Cheers...