TSXV:EVNI - Post Discussion
Post by
Wangotango67 on Mar 27, 2023 7:39pm
W-4 DRILL RESULTS READY ?
SODIUM PEROXIDE - 2Na202 is indifferent than, hydrogen peroxide
Once sodium peroxide is mixed with water it will
convert into - sodium hydroxide.
NICKEL SILICATES can host both oxide and chlroide - nickel
Once sodium peroxide is mixed into a flux
with ores and water is added - the water acts as
a catalyst to create a - fusion reaction.
Keep in mind - sodium peroxide with water
has now reacted and changed into sodium hydroxide
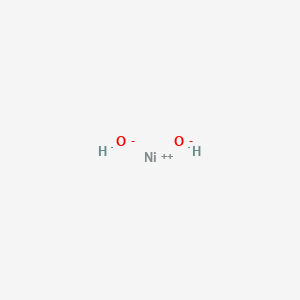
SODIUM HYDROXIDE - NaOH reaction with 3 types of nickel
- NiO + NaOH =
Ni(OH)2 + Na2O ( nickel hydroxide ) - NiCl2 + NaOH =
Ni(OH)2 + NaCl ( nickel hydroxide )
- NiS + NaOH = Ni(OH)2 + Na2S ( nickel hydroxide )
Based on thre above - it seems sodium peroxide is the " superb " oxidant to convert all silicate nickel
and nickel sulphide species into - nickel hydroxide. Punching in, sodium peroxide values into chemical
equation converters doesn't - factor in - the water inwhich
converts the " sodium peroxide flux ) into sodium hydroxide. H2O - is needed to complete the reaction equation. Ni(OH)2 - nickel hydroxide 2 nickel
2 oxygen
2 hydrogen
= 6 molecules
Not bad at all... only 6 molecules.
Yet... some research papers pointed out
a SODIUM bond can occur resulting in
nickel + sodium + oxygen + hydrogen
FAR LARGER MOLECULE
SOME RESEARCH PAPERS
Recommend -follow up with - HCl or Ntric acids.
Such would create larger complex bonds.
Larger the bond - less nickel in a droplette.
SOME RESEARCH PAPERS
Recommend - flux must be heated at specific temps
------------------------------------------------------------------------------
So.... sodium peroxide converts to sodium hydroxide
All nickel values ( kinds ) convert to a mono format of,
nickel hydroxide - i will not complain. I actually love it.
Unification allows better managemet of, nickel.
SO... WHAT WOULD BE THE IDEAL NEXT STEP ? ICP - AES vs ICP MS ICP AES does not detect - ions.
ICP MS - does detect - ions.
EVNI - 2011 assays used - ICP MS
EVNI - current assays used - ICP AES
CNC - assays used - ICP OES
This process does detect - ions.
------------------------------------------------------------------------------
MY OWN THOUGHTS -
Once nickle is in - hydroxide form -
One could....
- add more water to liberate any nickel with in flux paste
then drain solution with filter - add chlloride = electrolysis
all nickel would be accounted for via - electrolysis - add more water to liberate any nickel with in flux paste
then drain solution with filter - dehydrate and using
weighted sample value - test the sample for all nickel.
one would have all nickel accounted for - based on sample.
- add more water to liberate any nickel with in flux paste
then drain solution with filter - dehydrate additional water
leaving only a marginal amount in which then perform
ICP MS - looking for total ion count - with a compensation
factor of added water vs raw ore with no water
to determine actual - nickel value - in raw ores vs added water
which can skew a mineral value if - only a few mists are used
looking for an averaging vs testing entire sample weight value.
If agitated - few drops could provide an accurate value.
So.... there we have it.
Done with this subject.
A better understanding of, sodium peroxide.
Where " online fails " is not factoring in " water '
It's quite diff to find the end sum answer - due to
the equations omit - water.
They... don't speak about the " water catalyst "
turning the sodium peroxide into - sodium hydroxide.
At the end of the day....
it all revolves around - ICP AES vs ICP MS
MS - detects the ions.
EVNI use to use this format.
CNC - uses the OES format - which looks for ions.
I would say....
ion value is more - precise.
Thanks for bearing with me...
On the study of, assayig...
giggles.
Yes... i can be - persistent - just looking out for
junior + shareholders - looking for max value.
Hey, at least i found the switch up from,
ICP MS to ICP MS.
and that's a good thing.
I don't want to ever read up on assaying again...
lol - what a subject... ugh.
Are the W-4 drills ready ?
Hope so.
Be the first to comment on this post