Platinum Group assaying can be quite difficult. Especially when various Platinum's are in multibonds with other minerals.
Add an acid - will it break the bond or.... create compound bonds ?
70% Nickel 40% Platinum
Would the spectrometer light zero in on the 70% Nickel ?
Nickel Creek chose ( Arch assaying ) Samples were analyzed by 4-acid digestion and ICP-AES (ALS method MEICP-61)
with platinum, palladium and gold analysis by fire assay with
ICP-AES finish (ALS method PGM-ICP23). Selected massive sulphide samples are still being analyzed for the full PGE+gold suite by nickel sulphide collection fire assay with ICP-MS finish (ALS method PGM-MS25NS) 1 - what are those select results ?
2 - Why only sulphides ?
3 - Theme for Arch seems to only present sulphide Cu, Ni, with plats
4 - Fraction of, Hasen Creek ( formation )
sediment, limes, pebble conglomerates 5 - platinum group is highly stigmatized being in = sulphides.
6 - Lead sulphides with plats makes sense....but
7- There does exist (
platinum / palladium carbonate )
8 - If by chance Hazen Group is carbonate lime = should be further scoped for plat carbs
9 - Platinum is found in carbonates ( other world regions )
10- Carbonate Reefs
ICP AES FORMAT - FLAWS The great majority of analyses in ICP AES are carried out on liquid samples. The most convenient method for liquids to be introduced into the gas stream is as an aerosol from a nebulizer. Additionally, the performance of a particular nebulizer can be described using several attributes such as droplet size distribution, efficiency, stability, response time, tendency to clog and memory effects. https://www.sciencedirect.com/topics/materials-science/inductively-coupled-plasma-atomic-emission-spectroscopy
ALS Assaying ICP-AES (ALS method MEICP-61) 30 g sample vs 50 g higher detection Nearly 70% more mass using 50 g = higher odds of detection
4 acid =
not quite full digestion PGM-ICP23 Pt, Pd and Au by fire assay and ICP-AES finish.
30g sample PGM by lead collection fire assay
Homogenised and pulverised samples are mixed with flux composed of PbO and SiO2 with variable amounts of borax, soda ash and other reagents. The flux and sample are mixed, then heated at high temperature (>1,000°C) to decompose rock lattices and allow precious metals within the sample to be collected into a lead button. The button is placed in a porous cupel and heated again in an oxidising environment to convert lead to lead oxide that is absorbed into the cupel, leaving the precious metals behind as a dor bead or prill. The gold, platinum and palladium content of the prill is then determined spectroscopically.
https://www.alsglobal.com/en/geochemistry/precious-metals-analysis/platinum-group-elements
BORAX It lowers the fusion temperature for the formation of slags Platinum melting point ? 1,768 °C Lead is used in fire assaying for platinum and gold. Hmmmmm.
Majority of Wellgreens platinums are found in, Lead minerals. Fire assay temp - 1000 C
Borax reduces fusion
Platinum needs 1768 C
Question, how much platinum suites would precipitate into the Prill,?
How much platinum remains in the lead ?
Is this the best assay method ( more lead + borax )
When
Wellgreen Plats are mainly derived from lead ?
What does Nitric acid do by itself ? Solubilizes all other minerals - all but - platinum group.
This method leaves the plats behind = far easier
than adding more lead with insuffcient fire assay heat ( wink )
Highly recommend reading this research on, Platinum / Palladium Chemical History o f Palladium and Platinum.
B y R o b e r t K a n e MD MRIA
- platinum carbonate
- subset oxide group
- made me think of,
Hasen Creek formation ( sediment, limes ) - platinum group in carbonate phase ( might go undetected )
- most drill programs veer from this geology but
- some drill campaigns do drill this geology and
plats are present https://royalsocietypublishing.org/doi/pdf/10.1098/rstl.1842.0016
Hasen Creek ( geology ) Page 133 https://emrlibrary.gov.yk.ca/ygs/yeg/2004/2004_p129-146.pdf
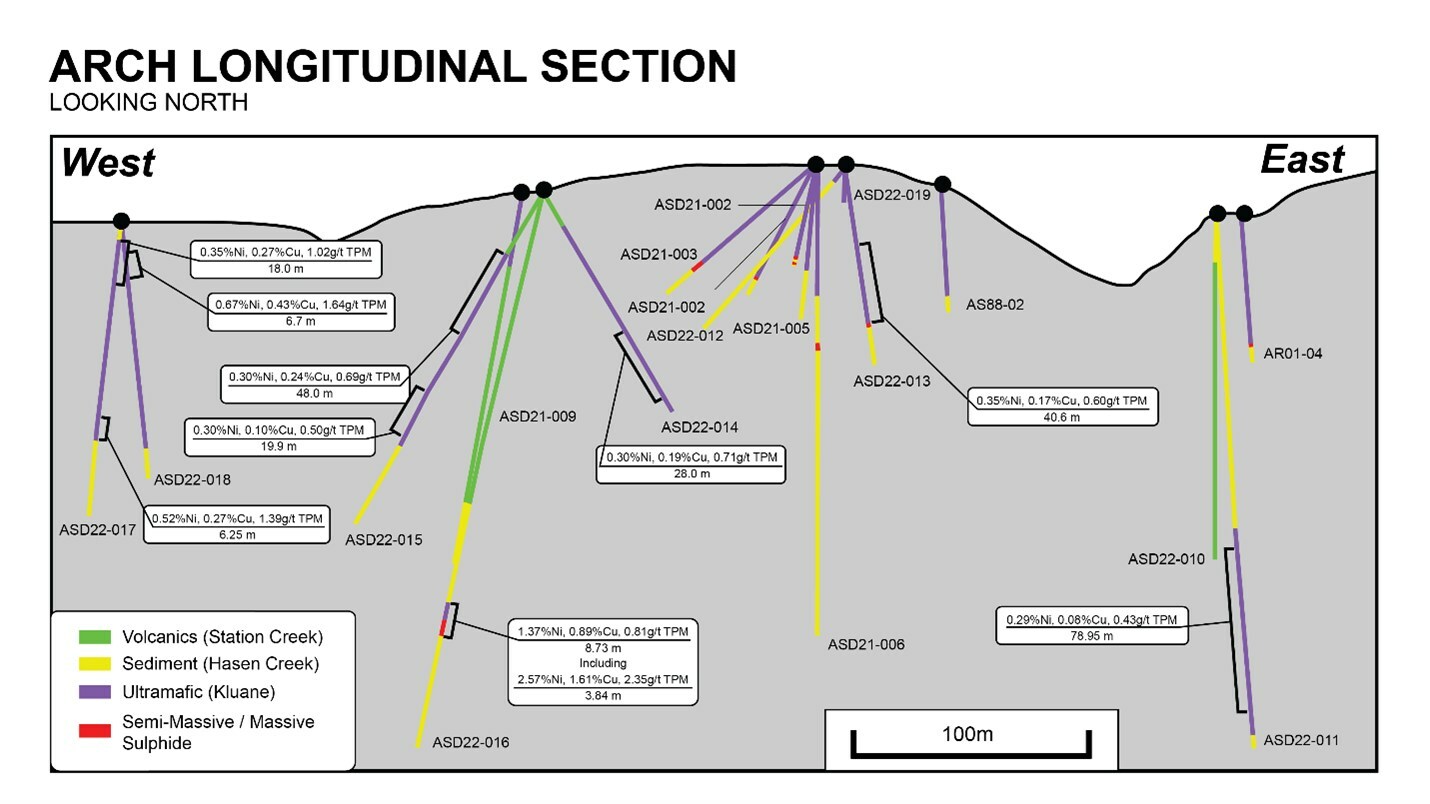
TIP ? Would anyone know the
full extent of Arch intercepts with this image below ?
lol
Arch has seen several meters drilling.
Of which, only one is revealed in image.
No one would know the great intercepts of, 2021, 2022
I thought 2021, 2022 intercepts were great.
Why are they not plotted ?
As bad as the new Corp presentation.
Can't see images in press ( all fuzzy ) and Corp is too small of print.
2021 drill results
https://s21.q4cdn.com/491660439/files/doc_downloads/2021/11/img1.jpg BELOW IMAGE - Notice ( more emphasis on sulphides )
copper, nickel sulphides.
Yet...
Hazen geology does present plat's. Historicals mention, plats at footwall ( contact zone ) of sediments.
Were Hasen,,sediment cores assayed or... only sulphides ?
Theory ? Sediment (carbonate limes ) do contain Plat's ( intercept seen in image )
Plats are there.
Hot sulphides come into contact converting carbonate plats to sulphide oxides ?
Would love to see a crew resift the cores and told - only seek plat 6 ( group )
Only assay for plats.
No lead fire assay
Just nitric - solubilize all other minerals.
Then take ( pulp ) and test with spectrometry.
Would plat 6 elements increase in percentage ?
 Wellgreen Minerals
Wellgreen Minerals A large variety of platinum-group minerals (PGM) are associated with base-metal sulfides in the Wellgreen Ni-Cu-PGE deposit, Yukon. Various solid-solutions occur in this deposit: (1) palladoan melonite - merenskyite - moncheite, (2) testibiopalladite - michenerite, (3) sudburyite - kotulskite - sobolevskite - (Ni,Pd)(Te,Sb,Bi)(1+chi) (palladoan imgreite - palladoan melonite), and (4) breithauptite - sudburyite, The associated PGM and PGE-bearing phases are sperrylite, stibiopalladinite or mertieite II (or both), geversite.
Pt-Pd-Fc-(Cu) alloys (Cu-Ni-rich
tetraferroplatinum, and
native platinum or
isoferroplatinum), froodite (?),
hollingworthite, laurite, native iridium, Rh-bearing cobaltite-gersdorffite, Pd-bearing ullmannite. and an unusual
Re-Ir-Os-Ru alloy. The following are important characteristics of the PGM in the Wellgreen deposit: the,,cry small gain-size of most of the PGM, unusually broad ranges of compositions of the
Pd-(Pt)-Ni-rich antimono- and bismuthotellurides,
a significant extent of Ni incorporation in the Pd-(Pt) antimono- and bismuthotellurides, and the presence of an uncommon and complex solid-solution involving
sudburyite, kotulskite, sobolevskite and Me(Te,Sb,Bi)(1+chi).
QUESTION ? Are spectrometry light machine / Nebulizers ( calibrated ) dialed in on,
Tetra bond Platinum Groups ?
Wink.
sobolevskite
Chemical Formula: Pb Bi ( uhmmm, Pd should be in this formula - lol )
Molecular Weight = 315.40 gm
Bismuth 66.26 % Bi
Palladium 33.74 % Pd
______
100.00 %
https://webmineral.com/data/Sobolevskite.shtml
kotulskite
Polyphase grain of (1) ferroan platinum corroded by (2) Pt(Fe,Cu) and overgrown by
(5) telluropalladinite, an unknown (6) Pd-Te-Fe-Si-O phase, (7) Fe-hydroxide and (8) a Ca-Mn-Fe silicate with inclusions
https://webmineral.com/cgi-bin/search/search.pl?Match=1&Realm=All&Terms=kotulskite
sudburyite
Chemical Formula: (Pd,Ni)Sb
Molecular Weight = 216.24 gm
Nickel 6.79 % Ni
Antimony 56.30 % Sb
Palladium 36.91 % Pd
______
100.00 %
https://webmineral.com/data/Sudburyite.shtml Here i thought i was done researching.
Thought i'd share...
May as well after seeing this stock trade with odd numer lots...lol
Boxing the price in slowly creating a, wall on the ask.
I'll stop posting now...
Giggles.
Hope a few readers found some of the topics interesting.
Cheers...