 As Stockhouse presents its multi-part series on Nash Pharmaceuticals (a wholly-owned subsidiary of Breathtec Biomedical Inc. (CSE: BTH, OTCQB: BTHCF, Forum), investors have had a chance to absorb this unique business model piece by piece. In the concluding installment, we connect the dots: huge revenue potential, real-world examples of this drug repurposing strategy in practice, and several key investment drivers.
As Stockhouse presents its multi-part series on Nash Pharmaceuticals (a wholly-owned subsidiary of Breathtec Biomedical Inc. (CSE: BTH, OTCQB: BTHCF, Forum), investors have had a chance to absorb this unique business model piece by piece. In the concluding installment, we connect the dots: huge revenue potential, real-world examples of this drug repurposing strategy in practice, and several key investment drivers.
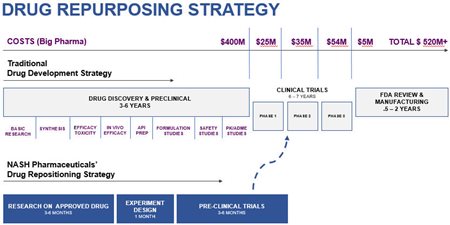
In Part 1, readers got the Big Picture. Via the Company’s “drug repurposing” strategy, Nash can bring old drugs to a patent-ready stage and into human clinical trials with maximum efficiency in terms of both time and cost.
This is due to the fact that the repurposed drugs at the heart of this new R&D have already been approved. This means much of the discovery and pre-clinical work has already been done. This can save up to 8 years in development time and potentially reduce costs by $10’s of millions.
(click to enlarge)
In Part 2, investors got important details on how Nash can execute on this strategy. Via Chief Scientific Officer, Dr. Mark Williams, the Company has identified 13 drugs that are potential repurposing candidates. With 7 of these, the data is already in place to commence fast-tracking into Phase II clinical studies.
Even more specifically, Nash has three R&D initiatives that it has targeted for immediate fast-tracking:
- Non-Alcoholic Steatohepatitis (NASH disease)
- Inflammatory Bowel Disease (IBD)
- Chronic Kidney Disease (CKD)
The significance of advancing to this stage has been explained, but for readers who missed the previous installments there are two important investment drivers here. Up to 50% of Phase II trials achieve positive results, with up to 33% of successful Phase II trials resulting in approved drugs [Source: PhRMA]. For this reason, such drug research already has significant commercial potential once R&D reaches this stage.
More efficient research for Nash = a faster payoff for shareholders. But this still misses the beauty of this business model.
Maximizing success/minimizing risk
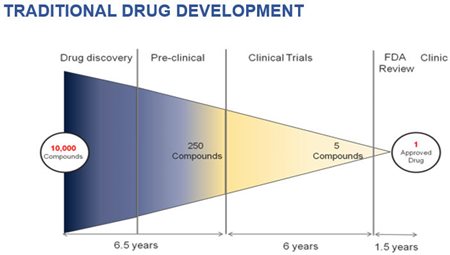
The Nash approach to pharmaceutical R&D is not merely a much faster/less expensive model to fast-track drug development. It also allows the Company to enter this R&D process when the odds of success are maximized.
(click to enlarge)
Roughly 90% of new drug research initiatives never even reach a Phase I study – where research shifts to actual testing on human patients. Not only does the Nash repurposing model chop out much of the time and expense for drug development, it also ratchets down the level of risk for both the Company and its shareholders.
Costs (and time) are minimized. The odds of success are maximized.
It’s a formula for pharmaceutical success. In Part 3, we see how drug repurposing (and drug “repositioning”) can become a payoff in the $100’s of millions for a junior pharma company. For the larger pharma companies that commercialize these drugs, the revenue streams can be in the billions of dollars.

(click to enlarge)
Biogen repurposed an (old) psoriasis drug into a (new) multi-billion treatment for multiple sclerosis.
Medivation repurposed an anti-histamine as a new treatment for Alzheimer’s disease and sold it for $400 million before completing a Phase III trial.
Celgene repositioned Thalidomide from being a (failed) morning sickness drug to a multi-billion dollar treatment for both leprosy and cancer.
Victoria-based Aspreva Pharmaceuticals repurposed a Roche Pharma drug for an “orphan indication” and then sold it for $915 million in 2007. At the time, it was a record for a made-in-Canada pharmaceutical product.
There are several take-aways here for investors. Generally speaking, we see how drugs previously developed for relatively mundane medical problems can be transformed into treatments for some of the most dangerous or debilitating medical conditions – with commensurate payoffs. However, the latter example is perhaps most instructive.
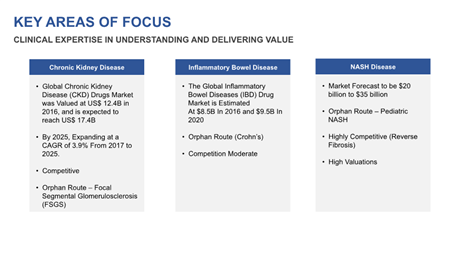
Like Aspreva, Nash also has great interest in orphan disease designations. Where no suitable therapy exists for a medical condition, the U.S. Food and Drug Administration (FDA) can designate a development-stage drug as “an orphan drug”, providing additional streamlining for R&D that can reduce time/costs even further.
Both Nash’s IBD research and CKD research have potential “orphan indications”. This means more than just greater efficiency. As perhaps the new standard of care for these diseases, there is fast-track commercialization potential in what are multi-billion dollar treatment markets.
(click to enlarge)
As exciting as those two initiatives are, most investors will be even more interested in Nash Pharmaceutical’s research into a new treatment for NASH disease. As noted in Part 2, the payoff for success here has already been telegraphed in a Reuters article from April 2017.
Large drugmakers with piles of cash are on the hunt for promising medicines being developed by small companies to treat NASH, a progressive fatty liver disease poised to become the leading cause of liver transplants by 2020. [emphasis mine]
That’s two lead research initiatives with orphan drug potential for the Company. However, those still take a back seat behind its NASH disease research, where the healthcare sector (and pharmaceutical industry) are clamoring for a new treatment to bring to market.
That clamor has never been louder. The Patent Cliff is the name the pharmaceutical industry has attached to a revenue-hole for Big Pharma that now totals in the $100’s of billions – due to the expiry of patents on a long list of major revenue producers.
With drug development far more expensive and time-consuming than ever before, Big Pharma has never been so eager to partner with junior pharma companies to replenish its pipeline. Here, astute investors will have already figured out an additional synergy in this business model.
Natural partners for commercialization
As noted, the pharmaceutical industry has a near-insatiable appetite today for new drugs that can be patented and commercialized. In the case of repurposing, however, particular drug companies will be especially interested in these new commercialization opportunities: the original drug developers.
With full infrastructure in place to manufacture these repurposed drugs along with existing distribution channels, acquiring repurposed drugs can be extremely lucrative for these companies. This translates into larger (potential) paydays for Nash and its shareholders. Not only might the original developers be willing to pay more for these repurposed drugs, they will also probably bid for them sooner.
If you’re the CEO of a drug-maker, watching a junior pharma company (like Nash) working to repurpose one of your drugs – with a new patent in play – the last thing you would want to see is one of your Big Pharma competitors jump in and grab that patent. Acquiring such drugs earlier and completing the R&D in-house (if necessary), could forestall a much more costly bidding war down the line.

(click to enlarge)
With Nash ready to commence fast-tracking on its lead initiatives, the Company plans on moving quickly. More news was just released on December 3, 2018: very strong results in pre-clinical work on its lead IBD initiative for Crohn’s disease. Not only did its lead compound (NP-178) exceed the current standard of care in some measures, Nash researchers have discovered a second compound (named “NP-120”) that delivered comparable results.
Drug repurposing. The Patent Cliff. Orphan drugs. Nash Pharmaceuticals. For tech investors looking to cash in on pharma opportunities, those first three terms will be invaluable in your due diligence. And after such education, the Nash business model will look even better.
breathtecbiomedical.com
FULL DISCLOSURE: Breathtec Biomedical Inc. is a paid client of Stockhouse Publishing.