
Government assistance has been a powerful force for mitigating the damage of COVID-19 in
several countries around the world. The state of Western Australia, for example, took a
precautious initiative with state-funded support networks to halt community spread — using
phone apps tracking data location and special guidelines for travellers entering the state. As of
March 11, Western Australia has only ten active cases — all of which are in quarantine or being
treated in hospital. Community transmission has rarely been a presence in the state.
Likewise, the Canadian Government has taken the steps to mitigate community transmission
with the use of contact tracing, testing sites, quarantine arrangements, travel risk assessment
at airports, and government funded medical initiatives. Although Canada isn’t blessed with the
watery isolation of Australia, the northern country has greatly reduced its number of cases from
January this year by over 6000. Canada has also now secured over 3.8 million doses of the
COVID-19 vaccine and is advised to enter the next phase of administering the medicine.
However, there will inevitably come a time when state-funded support networks will slowly roll
back to a diminished presence. This is a reality that will bloom when vaccinations are
administered to both the high and low-risk citizens, and community transmission slithers under
the cracks of society.
When this time comes, COVID-19 will not simply be forgotten though. Rather, its risks and
ability to transmit will be mitigated to a lower threat. As this happens, government intervention
will take the back seat to commercial products and the latest in medical technology. Businesses
that are already developing such methods to assist the public will step in with their products to
perform in a post-pandemic world.
Canada’s own
Therma Bright Incorporated (TSX-V: THRM, OTC: THRBF, Forum) is beginning to
ramp up the pipeline for its own patented product: AcuVid. AcuVid is designed to detect SARS-
CoV-2 (the COVID-19 causing virus) through saliva samples. Before the progressive medical
company can push AcuVid through hospitals, aged care facilities, schools, and entertainment
centres, it will need to tick some regulatory boxes.
Helping to put AcuVid on the ground floor, the company has secured an agreement with a
COVID-19 testing site in the greater Toronto area. Roughly 400 participants coming through the
location will be subject to AcuVid. Testing will begin this month.
"We are extremely pleased to have secured our first clinical test site in Ontario,” said Therma
Bright Chief Executive, Rob Fia. “Our simple, easy-to-use saliva-based test is very attractive for high volume users who want a scalable low-cost solution to keep their companies operating, entertainment venues open and schools and nursing homes safe,” he said.
This specific testing site is also a major win for the company as it sees a high volume of positive
cases. Therefore, Therma Bright expects to collect a wealth of data through a cost-efficient
manner.
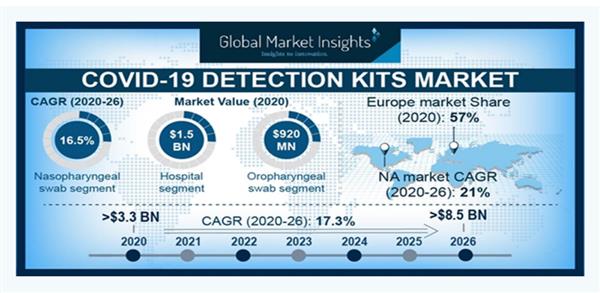
Once collected, the data will be passed onto the Food and Drug Administration and Health
Canada. For a home-use product to become legalised, it must satisfy an 80 per cent sensitivity
rate. AcuVid has achieved a rating of 86 per cent — putting it on the map for foreign interests.
“Recently, we have fielded many enquiries for our test from around the world,” Fia said.
Therma is wasting no time to take AcuVid across borders, having just filed its application for a
clinical trial with the Research Ethics Board (REB). The company will then follow up with REB
and Health Canada to begin the trials. Approval is expected by March 20.
Data from the trial will give the company the leverage to build AcuVid’s credibility in places
such as Europe.
"We are pleased to cross another hurdle in the process to get AcuVid to market,” Fia said.
Therma hopes to secure the ‘CE’ mark for commercial sale of AcuVid in Europe.
“Performance of the test during the development stage has exceeded our expectations,” he
continued.
“We are in the process of seeking initial product orders, subject to final regulatory approvals
and manufacturing scale up”.
In other news, Therma recently issued over 140,000 common shares to settle a $65,250 debt.
These shares are in a holding period, to expire on July 11.
Therma Bright is making no claims that AcuVid has the ability to cure the SARS-CoV-2 virus.
For more on the Company, visit
thermabright.com.
FULL DISCLOSURE: This is a paid article produced by Stockhouse Publishing.