(via Thenewswire.ca)
Toronto, Ontario / tnw-accesswire / December 23, 2014 / Theralase Technologies Inc. ("Theralase") (TLT:TSXV) (TLTFF: OTC Pink(R)) announced today that it has obtained preliminary results from the use of Photo Dynamic Therapy ("PDT") to destroy bladder cancer in an orthotopic ("occurring at the normal place in the body") rat model.
The University of Toledo in conjunction with the Princess Margaret Cancer Centre, University Health Network ("UHN") and Theralase conducted the experiment in a well-established orthotopic model for bladder cancer in rats.
Experiment:
-
-The rat bladder urothelium (inner lining) sustained localized injury via cauterization to allow adhesion of AY27 rat bladder cancer cells
-One of the lead Theralase Photo Dynamic Compounds ("PDCs") (TLD1433) was infused into the bladder once the tumour had reached 1 mm by 1 mm in size (Dose equal to 1 mg of PDC / 20 ml of distilled water solution)
-After 60 minutes incubation, the PDC was removed from the bladder and washed three times with distilled water
-The rats were separated into two groups:
-First group was used to assess preferential uptake; specifically, TLD1433 localization in healthy versus tumour urothelium
-Second group was used to assess PDT efficacy in the destruction of bladder cancer (150 mW / cm2 of visible red laser light for 30 minutes)
-To assess and quantify TLD1433 localization and efficacy in healthy versus tumour urothelium, Theralase employed visual inspection and Inductively Coupled - Plasma Mass Spectrometry ("ICP-MS")
Results:
-
-From the visual, clinical inspection, TLD1433 appeared to localize to specific regions of the bladder, indicating that TLD1433 accumulated in damaged and tumour urothelium
-ICP-MS analysis showed bulk tumour tissue accumulation of TLD1433 that was 180 times higher than in bladder wall of normal appearance, suggesting a preferential uptake in cancerous versus healthy urothelium, with a statistical significance of p< 0.01
-Areas of tumor necrosis were seen in treated bladders
-Post PDT, blood levels of TLD1433 following infusion were determined to be 50 nanograms of PDC per ml of solution using ICP-MS, which is 2000 times lower than the Maximum Tolerated Dose ("MTD")
Conclusions:
This data suggests that the combination of TLD1433 and visible red laser light has potential to induce urothelial tumour destruction with an exceptionally high margin of safety.
Discussion:
Theralase will continue evaluating these preliminary experiments to further confirm that laser light activated TLD1433 results in defined tumour necrosis with preservation of the normal bladder wall, sparing the surrounding urothelium. TLD1433 may thus offer versatility beyond what exists with traditional treatment methodologies for bladder cancer by destroying bladder cancer tumours and sparing healthy urothelium. TLD1433 is currently undergoing the final stages of pre-clinical optimization in preparation for a Health Canada and FDA human Phase I / IIa clinical study for this new class of PDCs in 2015.
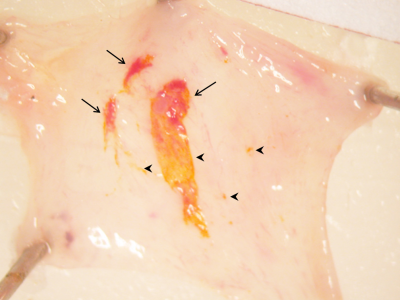
Click Image To View Full Size
-
-Tumour-bearing bladders were dissected after TLD1433 incubation and washing to assay localization to tumour tissue
-Arrows show co-localization of TLD1433 (bright yellow) with visually assayed tumour tissue (dark red)
-Arrowheads show TLD1433 localization without associated tumour, the significance of which is under study.
Steve Selman, MD, FACS, Chair and Professor of Urology and Director of Urological Research, University of Toledo, Toledo, Ohio stated that, "I am pleased to report that this preliminary study warrants further evaluation of the Theralase PDCs in their ability to localize to compromised urothelium and when laser light activated destroy the bladder cancer tumours via necrosis. This is early research, with non- optimized doses of PDC and laser light sources, but it is encouraging. Further evaluation with additional treated and control animals, along with additional blinded histopathological analysis will more definitely support these initial finding and provide us the scientific rigour required to strongly support out initial clinical findings."
Michael Jewett MD, FACS, Professor of Surgery in the Division of Urology at the University of Toronto and a member of the Department of Surgical Oncology at UHN stated that, "I am delighted that Theralase has advanced their research program to an animal model and that preliminary results have demonstrated preferential uptake of the PDC in cancerous urothelium. The healthy urothelium is not visibly affected and virtually no PDC was detected systemically, which are important elements of a high therapeutic index and favourable safety profile."
Roger Dumoulin-White, President and CEO of Theralase stated that, "I am elated that Theralase is able to report the successful achievement of this very significant milestone in our ongoing collaborative research and development program for our state-of-the-art PDC technology. It has always been Theralase's strategic milestone to prove the safety and efficacy of our PDC technology in an orthotopic rat model to provide our shareholders and investors the assurance that our technology has been validated to be safe and effective in animals prior to the Theralase team turning their attention to evaluating this technology in a human clinical study. I am proud to be associated with such a strong scientific and clinical team that was able to accomplish this very significant milestone. 2015 is going to be extremely exciting year for the Company."
About Theralase Technologies Inc.
Founded in 1994, Theralase Technologies Inc. ("Theralase(R)") (TSXV: TLT) (TLTFF: OTC Pink(R)) designs, manufactures and markets patented super-pulsed laser technology used for the elimination of pain, reduction of inflammation and dramatic acceleration of tissue healing. Theralase has sold over 1,200 systems to licensed healthcare practitioners, including: medical doctors, chiropractors, physical therapists and athletic therapists. Theralase has been so successful in healing nerve, muscle and joint conditions in clinical practice that Theralase's scientists and clinicians have now turned their attention to investigating the application of its lasers in the destruction of cancer, using specially designed molecules called Photo Dynamic Compounds ("PDCs"), which are able to localize to the cancer cells and when light activated, destroy them.
Additional information is available at www.theralase.com and www.sedar.com .
This press release contains forward-looking statements, which reflect the Company's current expectations regarding future events. The forward-looking statements involve risks and uncertainties. Actual results could differ materially from those projected herein. The Company disclaims any obligation to update these forward-looking statements.
Neither TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchanges) accepts responsibility for the adequacy or accuracy of this release.
For More Information:
Roger Dumoulin-White
President & CEO, Theralase Technologies Inc.
1.866.THE.LASE (843-5273) ext. 225
416.699.LASE (5273) ext. 225
rwhite@theralase.com
www.theralase.com
Copyright (c) 2014 TheNewswire - All rights reserved.