- First Antiretroviral Indicated to Reduce the Risk of HIV Infection in Adults in Canada -
MISSISSAUGA, ON, Feb. 29, 2016 /CNW/ - Gilead Sciences Canada, Inc. today announced that Health Canada has issued a Notice of Compliance for once-daily oral Truvada® (emtricitabine 200mg/tenofovir disoproxil fumarate 300mg) in combination with safer sex practices to reduce the risk of sexually acquired HIV-1 in adults at high risk, a strategy known as pre-exposure prophylaxis, or PrEP. This indication is based on clinical trials in men who have sex with men (MSM) at high risk for HIV-1 infection and in heterosexual serodiscordant couples. Truvada was originally approved in Canada in 2006 in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults.
"Multiple clinical trials have demonstrated that Truvada for PrEP is effective at reducing the risk of HIV infection acquired through sexual exposure," said Dr. Cécile Tremblay, Professor, Department of Microbiology, Infectious Diseases and Immunology, Université de Montréal, and infectious disease specialist, CRCHUM. "The number of new HIV infections in Canada has remained steady over the past several years and it is exciting to consider the potential impact of a new tool to help lower the rate of HIV infections in the future."
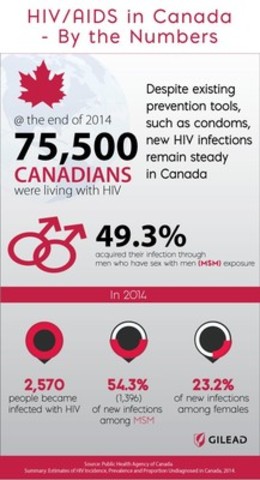
According to the Public Health Agency of Canada, in 2014, there were 2,570 new HIV infections reported in Canada, with 54.3 per cent (1,396) of the cases occurring among MSM and 23.2 per cent among females.
The approval is based on the results of two large placebo-controlled trials of Truvada for PrEP, the Pre-Exposure Prophylaxis Initiative (iPrEX) and Partners PrEP, sponsored by the U.S. National Institutes of Health (NIH) and the University of Washington respectively.
No new adverse reactions to Truvada were identified from the iPrEx and Partners PrEP trials, in which 2,830 HIV-1 uninfected adults received Truvada once daily for PrEP. The incidence and types of side effects were consistent with Truvada's safety and tolerability profile when used as an HIV treatment.
"Today's approval of Truvada for PrEP in Canada represents a meaningful advance in Gilead's efforts to address HIV across the entire spectrum, including prevention, treatment, and testing and linkage to care," said Norbert W. Bischofberger, PhD, Gilead's Executive Vice President, Research and Development and Chief Scientific Officer. "We are pleased to offer this important HIV prevention tool to at-risk populations in Canada and we remain engaged with regulatory agencies in other countries around the world that express interest in Truvada for PrEP."
Truvada was approved for PrEP in the United States in 2012, and in Kenya and South Africa in 2015. Regulatory submissions are pending in Australia, Brazil, Peru and Thailand. The European Medicines Agency (EMA) has fully validated Gilead's Type II variation application for Truvada, and it is now under evaluation by the EMA. Additionally, within the European Union, Truvada for PrEP is currently available in France following a Temporary Recommendation for Use by the French regulatory agency (ANSM).
Important Safety Information
Truvada used for a PrEP indication must only be prescribed to individuals confirmed to be HIV-negative immediately prior to initial use and periodically (at least every 3 months) during use. Drug-resistant HIV-1 variants have been identified with the use of Truvada for a PrEP indication following undetected acute HIV-1 infection. Do not initiate Truvada for a PrEP indication if signs or symptoms of acute HIV infection are present unless negative infection status is confirmed. For important safety information for Truvada for PrEP, including contraindications and additional serious warnings and precautions, please see the Canadian Product Monograph for Truvada available at www.gilead.ca.
About Gilead Sciences
Gilead Sciences is a biopharmaceutical company that discovers, develops and commercializes innovative therapeutics in areas of unmet medical need. The company's mission is to advance the care of patients suffering from life-threatening diseases. Gilead has operations in more than 30 countries worldwide, with headquarters in Foster City, California. Gilead Sciences Canada, Inc. is the Canadian affiliate of Gilead Sciences, Inc. and was established in Mississauga, Ontario in 2005.
Forward-Looking Statement
This press release includes forward-looking statements, within the meaning of the Private Securities Litigation Reform Act of 1995 that are subject to risks, uncertainties and other factors, including the risks that physicians and patients may be reluctant to use Truvada for HIV risk reduction. As a result, there may not be significant use of Truvada as a risk reduction tool. These risks, uncertainties and other factors could cause actual results to differ materially from those referred to in the forward-looking statements. The reader is cautioned not to rely on these forward-looking statements. These and other risks are described in detail in Gilead's Annual Report on Form 10-K for the year ended December 31, 2015, as filed with the U.S. Securities and Exchange Commission. All forward-looking statements are based on information currently available to Gilead, and Gilead assumes no obligation to update any such forward-looking statements.
Canadian Product Monograph for Truvada is available at www.Gilead.ca.
Truvada is a registered trademark of Gilead Sciences, Inc., or its related companies
For more information on Gilead Sciences, please visit the company's website at www.gilead.com, follow Gilead on Twitter (@GileadSciences) or call Gilead Public Affairs at 1-800-GILEAD-5 or 1-650-574-3000
SOURCE Gilead Sciences, Inc.
Image with caption: "HIV/AIDS in Canada - By the Numbers (CNW Group/Gilead Sciences, Inc.)". Image available at: http://photos.newswire.ca/images/download/20160229_C1401_PHOTO_EN_630919.jpg

Patrick O'Brien, Investors, +1 (650) 522-1936; Ryan McKeel, Media, +1 (650) 377-3548Copyright CNW Group 2016