(via TheNewswire)
Edmonton, Alberta - TheNewswire - (August 29th, 2019) - BioNeutra Global Corporation ("BioNeutra" or the "Company") (TSXV:BGA) reports on the financial results for the second financial quarter ended June 30th, 2019.
FINANCIAL HIGHLIGHTS
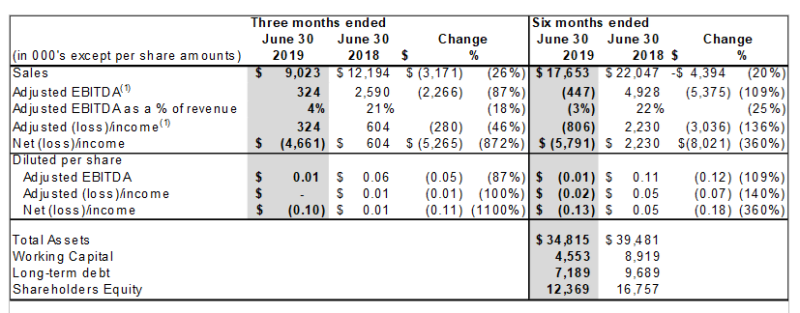
Click Image To View Full Size
- (1)Refer to the "Non-IFRS Measures" section for a definition of non-GAAP terms as well as reconciliations for Adjusted EBITDA, Adjusted operating (loss)/income, and Adjusted (Loss)/income.
Q2 2019 and YTD HIGHLIGHTS
BioNeutra continued its market penetration obtaining new distribution agreements and further expanded its product offering geographically. Despite this effort, the Company was faced with a series of challenges that impact sales, gross profit and EBITDA during the quarter. Supply chain shortages, product warranty claim, inventory impairment provision, increased tariff charges all negatively impacted the Company's profitability for the second quarter of 2019.
BioNeutra Canadian manufacturing plant is presently commissioned, licensed and in production. As a result, we are now positioned to provide high quality retail products from the Canadian facility. In addition, the Canadian plant positions us to help offset possible future adverse events that may arise in other contracted plants at other locations. The plant has not yet reached full capacity and as such costs per kilo in quarter 2 were considerably higher than budgeted. Management has taken steps to increase production and lower costs for the second half of 2019.

Sales for the three-month period June 30, 2019 were $9,022,745, compared to sales of $12,193,507 for the same period of 2018 a decrease of $3,170,672 or 26%.

Sales for the six-month period ended June 30, 2019 were $17,653,111, compared to $22,046,553, for the first half of 2018, a decrease of $4,393,442 or 20%. The decrease primarily relates to one customer curtailing purchases as they adjusted for their new packaging and labeling requirements.
Adjusted EBITDA for the second quarter was $124,000 compared to $2,589,000 in the comparable period in 2018. This 95% decrease was to due lower margins as the Company ran into a product manufacturing shortage from one of our contact manufacturing resulting in us substituting a higher costed ingredient and selling that product below cost. In addition, the Company recorded an inventory impairment of $ 1,376,176, a bad debt allowance of $829,985 and contingent liability for product warranty claims for $947,427.
The Company recorded an adjusted net loss of $19,000 and $1,149,000 or $0.00 and negative $0.03 diluted loss per share for the three and six months ended June 30, 2019.
BioNeutra had working capital of $4,553,012 compared to $8,919,230 at June 30, 2018, a decrease of 49%. This was due to the increase in inventory based on increased demand for product and the Company increasing its infrastructure costs to ensure it can meet demand requirements.
The Company's products that are being sold are becoming more and more diversified, from original basic VitaFiber powder and syrup to current organic, DP3, organic DP3, VitaSweet, DexiFiber, Beta Glucan, BetaFiber powder and syrup, as well retails products. The Company's sales regions and countries are continually further expanded to more countries in the world, new regions recently explored include India, Thailand, Indonesia, Malaysia, Vietnam, Cambodia, Philippines, Australia & New Zealand, Turkey, Israel and Brazil.
OUTLOOK
Management has taken proactive steps to respond to the critical shortage of supply from contract manufacturers by initiating new contract agreements and developing plans to expand capacity of manufacturing that would be owned by BioNeutra. As noted, this critical outage of supply means that margins in the second quarter for several of our main products were severely suppressed or even at times at a negative percentage. BioNeutra chose to protect our customers from shortages by substituting high cost product. We believe that this situation will occur to some degree into the third quarter.
BioNeutra is continuing its path to obtain market share in regions where it currently provides products, namely North America, Europe, India and South East Asia. Our retail products are being revamped and are planning a new launch in September of this year. Our ability to produce quality products at traditional gross profit is currently dependent on our contracting manufacturing and we will seek to have multiple contracts to minimize supply chain risk.
The Company has continued to invest in research and development as a commitment to our quality, but also to create new products that can complement our existing product offering.
Industry trends in health and wellness, plant-based products, fiber and sugar all strongly support continued growth and demand for our product. A key feature of VitaFiber IMOs is their versatility. Few competitive products, and none manufactured in North America, possess the full functionality of these products. Its unique combination of health attributes allows BioNeutra to enter several segments within both mainstream and functional food and beverage markets and retail businesses. Coupled with superior functional attributes and extensive regulatory approvals, VitaFiber IMO represents excellent value for food and beverage manufacturers. They are also available as a certified organic product.
About BioNeutra
BioNeutra is in the business of research and development, production and commercialization of ingredients for nutraceutical, functional and mainstream foods and beverages, with a focus on VitaFiber(TM) IMOs.
The Company's lead product, VitaFiber(TM), is an advanced functional and health food and beverage ingredient scientifically made from natural agricultural products, is generally regarded as safe (GRAS) by the U.S. Food & Drug Administration, and is European Food Safety Authority and Health Canada approved as a novel food ingredient. VitaFiber(TM) is naturally sweet and lower in calories than regular sugar and is a natural source of dietary fiber as it provides low calorie soluble prebiotic fiber for human digestive health.
The Company produces VitaFiber(TM) using its patented processes that naturally transform starch molecules from agriculture cereal crops including Non-GMO corn, wheat, barley, potato, or tapioca into the functional health molecules of VitaFiber(TM) IMO. The VitaFiber(TM) manufacturing process is based upon a natural enzymatic conversion of starch molecules without any chemical modification involved, making VitaFiber(TM) a natural food and beverage ingredient. VitaFiber(TM) is also non-GMO, vegan-friendly, gluten-free, Kosher and Halal certified and available as certified organic.
The Company's customers include a mix of small and medium enterprises and a number of high-profile food and beverage manufacturers in Canada, the U.S., Europe, the United Kingdom, and Mexico. VitaFiber(TM) is also available for retail purchase across the globe through Amazon.com and other direct-to-consumer retailers.
Further information about BioNeutra is available on the Company's website at www.bioneutra.ca and the SEDAR website at www.sedar.com.
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
For further information on BioNeutra, please contact:
Dr. Jianhua Zhu
President and Chief Executive Officer
Tel: (780) 466-1481 (Ext. 132)
Email: jianhua.zhu@bioneutra.ca
Forward-Looking Information
This press release may include forward-looking information within the meaning of Canadian securities legislation concerning the business of BioNeutra. Forward-looking information is based on certain key expectations and assumptions made by the management of BioNeutra. Although BioNeutra believes that the expectations and assumptions on which such forward-looking information is based are reasonable, undue reliance should not be placed on the forward-looking information because BioNeutra can give no assurance that they will prove to be correct. Forward-looking statements contained in this press release are made as of the date of this press release. BioNeutra disclaims any intent or obligation to update publicly any forward-looking information, whether as a result of new information, future events or results or otherwise, other than as required by applicable securities laws.
This news release does not constitute an offer to sell or a solicitation of an offer to buy any of the securities described herein in the United States. The securities described herein have not been and will not be registered under the United States Securities Act of 1933, as amended, or any applicable securities laws or any state of the United States and may not be offered or sold in the United States or to the account or benefit of a person in the United States absent an exemption from the registration requirements.
Copyright (c) 2019 TheNewswire - All rights reserved.