NEW HAVEN, Conn., Sept. 10, 2019 /PRNewswire/ -- Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN), a biotechnology company focused on advancing innovative therapies for neurological and neuropsychiatric diseases, today announced completion of enrollment in its Phase 2/3, double-blind, randomized, placebo-controlled, dose-ranging trial of intranasally administered vazegepant (formerly BHV-3500) for the acute treatment of migraine. Vazegepant is a novel, structurally unique, third-generation calcitonin gene-related peptide (CGRP) receptor antagonist and the second product candidate in development for the acute treatment of migraine from Biohaven's NOJECTION™ Migraine Platform.
The Phase 2/3 trial is designed to measure efficacy on regulatory endpoints for acute treatment of migraine – pain freedom and freedom from the most bothersome migraine-associated symptom at 2 hours post-dose – for vazegepant across three doses (5 mg, 10 mg and 20 mg) versus placebo. The trial will also study other clinical measures considered critically important to people living with migraine, such as pain relief and the return to normal functioning.
Elyse Stock, M.D., CMO of Biohaven, commented, "Biohaven is proudly committed to innovating new treatment options for people living with the debilitating condition of migraine. Our Phase 2/3 trial of vazegepant represents the first late-stage study of an intranasally delivered CGRP receptor antagonist, propelling us a step closer to providing patients with multiple, easy-to-use formulations to treat migraine." Dr. Stock added, "Vazegepant is complementary to our lead migraine asset, rimegepant, which has met the primary efficacy endpoints in three completed Phase 3 clinical trials and is currently under review with the FDA."
Intranasal vazegepant utilizes the Aptar Pharma Unidose System (UDS), which is designed to enable systemic delivery of drugs without the need for injection or administration by a healthcare professional. This device is FDA-approved to deliver multiple drug products marketed in the U.S., and is used by thousands of people every day.
Robert Croop, M.D., Biohaven's Chief Development Officer – Neurology, added, "We are excited to complete enrollment with vazegepant, our third-generation CGRP receptor antagonist, in this pivotal Phase 2/3 trial with the goal of ultimately providing patients with important new treatment options that can easily be self-administered whenever and wherever a migraine strikes."
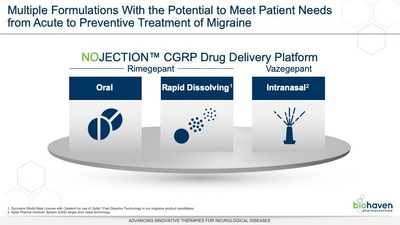
Vazegepant is the second of Biohaven's CGRP receptor-targeting compounds to enter clinical trials. Biohaven's multiple CGRP receptor antagonist product candidates, including rimegepant, and expanded array of formulations including intranasal delivery and Catalent's Zydis® oral fast-dissolve tablet, are designed to meet patients' needs across the spectrum from acute to preventive treatment of migraine.
About Migraine
Over 36 million Americans suffer from migraine. Migraine attacks can differ in intensity and frequency, with many being highly disabling. More than 90 percent of migraine sufferers are unable to work or function normally during an attack. In the Global Burden of Disease Study, updated in 2015, migraine was ranked as the seventh highest cause worldwide of years lost due to disability. CGRP receptor antagonists represent a novel class of drug candidates for the treatment of migraine and are the first new class specific to the acute treatment of migraine in over 25 years. This unique and specific mode of action potentially offers an alternative to current agents, particularly for patients who have contraindications to the use of triptans, such as those with underlying cardiovascular diseases, or who either do not respond or have inadequate or inconsistent response to triptans or are intolerant to them.
About Biohaven
Biohaven is a clinical-stage biopharmaceutical company with a portfolio of innovative, late-stage product candidates targeting neurological diseases, including rare disorders. Biohaven has combined internal development and research with intellectual property licensed from companies and institutions including Bristol-Myers Squibb Company, AstraZeneca AB, Yale University, Catalent, Rutgers, and ALS Biopharma LLC. Currently, Biohaven's lead development programs include multiple compounds across its CGRP receptor antagonist, glutamate modulation, and myeloperoxidase inhibitor platforms. Biohaven's common shares are listed on the New York Stock Exchange and traded under the ticker symbol BHVN. More information about Biohaven is available at www.biohavenpharma.com.
About Aptar Pharma
Aptar Pharma is part of AptarGroup, Inc. (NYSE: ATR), a leading global supplier of a broad range of innovative dispensing, sealing and active packaging solutions for the beauty, personal care, home care, prescription drug, consumer health care, injectables, food and beverage markets. Aptar uses insights, design, engineering and science to create innovative packaging technologies that build brand value for its customers, and, in turn, make a meaningful difference in the lives, looks, health and homes of people around the world. Aptar is headquartered in Crystal Lake, Illinois and has over 14,000 dedicated employees in 18 different countries. For more information, visit www.aptar.com/pharma. Media Contact: Carolyn Penot, Aptar Pharma, +33 1 39 17 20 38, carolyn.penot@aptar.com
Forward-Looking Statements
This news release includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements involve substantial risks and uncertainties, including statements that are based on the current expectations and assumptions of the Company's management. All statements, other than statements of historical facts, included in this press release, including statements about the potential safety, efficacy and attractive mode of administration of vazegepant as a treatment for migraine, as well as its potential for ultra-rapid onset and sustained activity, the potential of the Company's CGRP receptor antagonist drug candidates to provide an improved, effective and safe treatment option for the acute and preventive treatment of migraine and the Company's expected timelines for receipt of data from clinical trials, are forward-looking statements. The use of certain words, including "believe," "potential" and "will" and similar expressions, is intended to identify forward-looking statements. The Company may not actually achieve the plans and objectives disclosed in the forward-looking statements, and you should not place undue reliance on the Company's forward-looking statements. Various important factors could cause actual results or events to differ materially from those that may be expressed or implied by our forward-looking statements, including those described in the "Risk Factors" section of the Company's Annual Report on Form 10-K filed with the Securities and Exchange Commission on February 28, 2019, the Company's Quarterly Report on Form 10-Q for the quarter filed with the Securities and Exchange Commission on August 9, 2019, and other filings Biohaven makes with the U.S. Securities and Exchange Commission from time to time. The forward-looking statements are made as of this date and the Company does not undertake any obligation to update any forward-looking statements, whether as a result of new information, future events or otherwise, except as required by law.
For further information, contact Dr. Vlad Coric, Chief Executive Officer, Biohaven at Vlad.Coric@biohavenpharma.com
 View original content to download multimedia:http://www.prnewswire.com/news-releases/biohaven-advances-nojection-migraine-platform-with-completion-of-enrollment-of-pivotal-phase-23-trial-of-vazegepant-the-first-intranasally-administered-cgrp-receptor-antagonist-in-development-for-the-acute-treatment-of-migraine-300914911.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/biohaven-advances-nojection-migraine-platform-with-completion-of-enrollment-of-pivotal-phase-23-trial-of-vazegepant-the-first-intranasally-administered-cgrp-receptor-antagonist-in-development-for-the-acute-treatment-of-migraine-300914911.html
SOURCE Biohaven Pharmaceutical Holding Company Ltd.