VenoValve Patients Continue to Improve at 6 Months
IRVINE, CA / ACCESSWIRE / October 24, 2019 / Hancock Jaffe Laboratories, Inc. (NASDAQ:HJLI)(NASDAQ:HJLIW), a developer of medical devices that restore cardiac and vascular health, announced today that Dr. Jorge Hernando Ulloa, the Principal Investigator for HJLI's first-in-human VenoValve study in Bogota, Colombia, presents new six (6) month VenoValve data at the 20th Congress of Asian Society for Vascular Surgery in Bali, Indonesia.
Dr. Ulloa's presentation indicates that each of the first five (5) VenoValve patients continued to improve in all study end-points at six (6) months post VenoValve surgical implant, including average improvements of an additional ten (10) percentage points in reflux, two (2) percentage points in disease manifestations (VCSS scores), and thirteen (13) percentage points in pain (VAS scores), compared to levels at ninety (90) days post VenoValve surgeries. At six (6) month post VenoValve implant surgery, improvements for all five (5) patients when compared to Pre-VenoValve levels are as follows:
| | Reflux - Improvement | VCSS - Improvement | VAS - Improvement |
Patient 1 | 80% | 63% | 75% |
Patient 2 | 30% | 60% | 56% |
Patient 3 | 100% | 67% | 50% |
Patient 4 | 65% | 38% | 50% |
Patient 5 | 30% | 69% | 38% |
AVERAGE | 61% | 59% | 54% |
Patient 5, who was previously thought to have occluded, continues to improve and may have at least partial functionality of her VenoValve. Dr. Ulloa also provides initial data on the improvements of VenoValve patient 6, who is ninety (90) days post VenoValve surgery, and the improvements on VenoValve patients 7 and 8, who are sixty (60) days post VenoValve surgeries, which are as follows:
| | Reflux - Improvement | VCSS - Improvement | VAS - Improvement |
Patient 6 | 60% | 50% | 75% |
Patient 7 | 73% | 76% | 70% |
Patient 8 | 35% | (17%) | 38% |
Dr. Ulloa's presentation also includes before and after pictures of a venous ulcer, from which the patient suffered for more than eighteen (18) months, and that was completely healed at sixty (60) days post VenoValve surgery (see image).
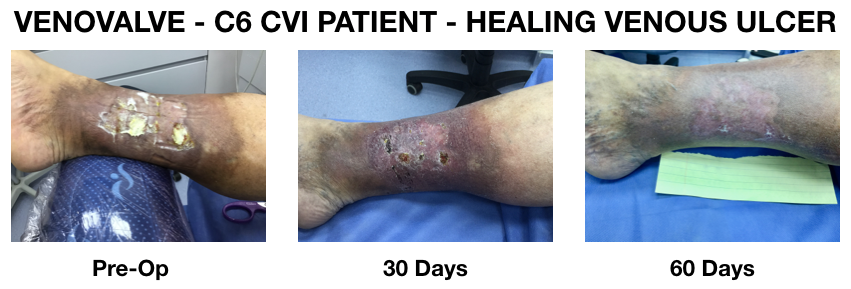
In addition, Dr. Ulloa's presentation indicates that a ninth (9th) VenoValve patient was enrolled in the study and successfully implanted in October. Select slides from Dr. Ulloa's presentation, including the before and after pictures of the venous ulcer, are available at https://ir.hancockjaffe.com/ir-calendar/detail/2304/venovalve-6-month-data. Safety issues for the reporting periods have been relatively minor, including one (1) fluid pocket (which was as aspirated), intolerance from Coumadin anticoagulation therapy, and two (2) minor wound infections (treated with antibiotics).
Robert Berman, Hancock Jaffe's Chief Executive Officer stated, "If our patients had merely maintained their ninety (90) day improvement levels, that would have been a positive result. Instead, our patients have continued to show improvement. What is equally as impressive is the extensive healing of the venous ulcer at only sixty (60) days post VenoValve surgery. Our goal with the VenoValve is to improve the lives of patients with CVI and this data and the pictures show that we are on the right path."
The first-in-man Colombian study will initially include ten (10) patients who suffer from severe, chronic venous insufficiency (CVI) of the deep vein system. CVI occurs when the valves in the veins of the leg are injured or destroyed, causing blood to flow backwards, which is known as reflux. Reflux results in increased venous pressure (venous hypertension), damage to the veins, and results in the pooling of blood in the lower leg. Deep venous CVI is a serious condition, often resulting in debilitating pain, swelling, and open sores (venous ulcers) on the lower leg.
Dr. Marc H. Glickman, Hancock Jaffe's Senior Vice President and Chief Medical Officer stated, "It is gratifying to be able to help patients that are in such distress and have no other effective treatment options. The venous ulcers that we typically encounter are extremely painful, and negatively impact all aspects of the patients' lives. The results that we are seeing so far in terms of ulcer healing are beyond my expectations."
Next steps for the VenoValve include the enrollment and implantation of the tenth (10th) patient, the continual monitoring of all VenoValve recipients, reengagement with the U.S. Food and Drug Administration ("FDA"), presentation of the six (6) month data to potential strategic partners, and a series of FDA mandated testing on the final VenoValve design, all of which are in preparation for the filing of an IDE application with the FDA for the U.S. pivotal trial.
Approximately 2.4 million people in the U.S. suffer from CVI due to reflux in the deep venous system, representing the potential for recurring revenue of hundreds of millions of dollars per year. Estimates indicate that direct medical costs from CVI in the U.S. exceed $30 Billion a year. There are currently no FDA approved devices, or effective treatments for deep venous CVI.
About Hancock Jaffe Laboratories, Inc.
Hancock Jaffe Laboratories (NASDAQ: HJLI) specializes in developing and manufacturing bioprosthetic (tissue based) medical devices to establish improved standards of care for treating cardiac and vascular diseases. Hancock Jaffe currently has two lead product candidates: the VenoValve®, a porcine based valve which is intended to be surgically implanted in the deep venous system of the leg to treat reflux associated with Chronic Venous Insufficiency; and the CoreoGraft®, a bovine tissue based off the shelf conduit intended to be used for coronary artery bypass surgery. Hancock Jaffe has a 20-year history of developing and producing FDA approved medical devices that sustain or support life. The current management team at Hancock Jaffe has been associated with over 80 FDA or CE marked medical devices. For more information, please visit HancockJaffe.com.
Cautionary Note on Forward-Looking Statements
This press release and any statements of stockholders, directors, employees, representatives and partners of Hancock Jaffe Laboratories, Inc. (the "Company") related thereto contain, or may contain, among other things, certain "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements involve significant risks and uncertainties. Such statements may include, without limitation, statements identified by words such as "projects," "may," "will," "could," "would," "should," "believes," "expects," "anticipates," "estimates," "intends," "plans," "potential" or similar expressions. These statements are based upon the current beliefs and expectations of the Company's management and are subject to significant risks and uncertainties, including those detailed in the Company's filings with the Securities and Exchange Commission. Actual results (including, without limitation, the performance of the new board members described herein) may differ significantly from those set forth or implied in the forward-looking statements. These forward-looking statements involve certain risks and uncertainties that are subject to change based on various factors (many of which are beyond the Company's control). The Company undertakes no obligation to publicly update any forward-looking statements, whether as a result of new information, future presentations or otherwise, except as required by applicable law.
HJLI Press Contact:
Amy Carmer
Tel: 949-261-2900
Email: ACarmer@HancockJaffe.com
Media & Investor Relations Contact:
MZ North America
Chris Tyson
Managing Director
(949) 491-8235
HJLI@mzgroup.us
www.mzgroup.us
SOURCE: Hancock Jaffe Laboratories, Inc.
View source version on accesswire.com:
https://www.accesswire.com/564086/Principal-Investigator-Dr-Jorge-Hernando-Ulloa-Presents-6-Month-VenoValve-Data-at-the-20th-Congress-of-Asian-Society-for-Vascular-Surgery
