NEWARK, Calif., Dec. 3, 2019 /PRNewswire/ -- Protagonist Therapeutics, Inc. (Nasdaq:PTGX) today announced preliminary results from patients with transfusion-dependent (TD) beta-thalassemia in the ongoing TRANSCEND Phase 2 open-label study of PTG-300. Dose-related drug exposure and reductions from baseline serum iron and transferrin saturation (TSAT) levels were observed, with significant reductions at the 40 mg and 80 mg weekly doses (p < 0.02 for each assessment, see graphs below).
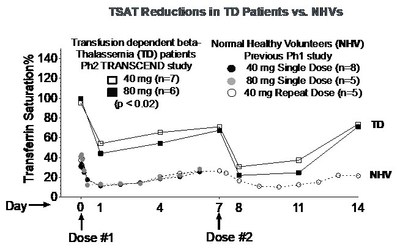
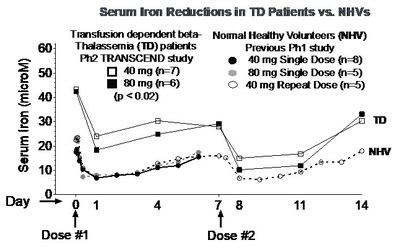
"The dose-related pharmacodynamic responses in serum iron and TSAT levels observed in this preliminary analysis provide the first evidence of the effects of PTG-300 in patients with beta-thalassemia, who have highly elevated levels of iron in the body," commented Samuel Saks, M.D., Protagonist Chief Medical Officer. "These early results suggest the potential of finding an appropriate dose of PTG-300 for continued development in the treatment of beta-thalassemia. While we have observed clinical responders in the study based on the pre-specified criteria of reductions in transfusion burden, continued evaluation at higher doses will be required to evaluate the rate and durability of these effects in order to reach definitive conclusions. We look forward to the results from further study with additional dose regimens and longer follow-up, with clinical efficacy results expected in 2020."
"Treatment options for patients with beta-thalassemia are limited and the complications associated with transfusion are serious," commented Ashutosh Lal, M.D., Program Director of the Comprehensive Thalassemia Center at the UCSF Benioff Children's Hospital, Oakland, and PTG-300 beta-thalassemia study investigator. "The TRANSCEND trial is examining whether constraining iron availability improves endogenous hemoglobin synthesis in patients with beta-thalassemia, an endpoint for which there exists considerable preclinical evidence. These results demonstrating the pharmacodynamic activity of PTG-300 in reducing TSAT, though preliminary, warrant continued evaluation of PTG-300 for the potential treatment of beta-thalassemia."
"The consistent and significant effect on iron levels observed in normal healthy volunteers in a previous study, and now in patients with beta-thalassemia, provides strong rationale for potential utility of PTG-300 in blood disorders directly dependent on disruption of normal iron homeostasis in the body," commented Dinesh V. Patel, Ph.D., Protagonist President and Chief Executive Officer. "We are encouraged by these findings and are continuing with our original plans of conducting clinical proof-of-concept studies with PTG-300 in different blood disorders such as beta-thalassemia, polycythemia vera, hereditary hemochromatosis, and an investigator sponsored study in myelodysplastic syndromes. We are well financed to conduct these studies and our corporate objective is to make data-driven decisions in 2020, with the intent of choosing our first clinical indication for a potential pivotal study to begin in 2021."
In the study, PTG-300 was well-tolerated and systemic adverse events were mild to moderate in severity and were typical of patients with TD beta-thalassemia. These events were not dose-related and did not prevent dose escalation. There was one serious adverse event of vomiting and confusion, and the most frequent treatment emergent adverse event observed was transient erythema in 4 out of 33 patients (12 percent).
Conference Call and Webcast Information
Protagonist will host a conference call at 8:30 a.m. EST today, Dec. 3, 2019. To access the live call, dial 1-844-515-9178 (U.S./Canada) or 1-614-999-9313 (international) and refer to conference ID number 1662899. The call will also be webcast and will be accessible from "Events & Presentations" in the Investors section of the Company's website at www.protagonist-inc.com. A replay will be available on the Company's website following the call.
About the Phase 2 TRANSCEND Study
The global Phase 2 study is a single-arm, open label, multiple-ascending dose design that will evaluate safety, proof-of-concept and dose finding in adolescent and adult patients with anemia associated with non-transfusion-dependent (NTD) or transfusion-dependent (TD) beta-thalassemia. NTD patients will receive 12 weeks treatment with PTG-300 in escalating dose cohorts. The primary efficacy endpoint in NTD patients will be change in hemoglobin from baseline. TD patients will receive 16 weeks treatment with PTG-300 in escalating dose cohorts. The primary efficacy endpoint in TD patients will be a change in transfusion burden from baseline. All patients completing the trial will have the opportunity to participate in an open-label extension for two years. Additional information on the PTG-300 beta-thalassemia study is available at https://clinicaltrials.gov/ct2/show/NCT03802201.
About PTG-300
PTG-300 is an injectable hepcidin mimetic in clinical development for the potential treatment of beta thalassemia and polycythemia vera. Hepcidin is a natural peptide hormone that is a critical regulator governing iron absorption, recycling and utilization by the body. Iron plays an essential role in various body functions, especially blood formation. Excess iron in the body is toxic, resulting in tissue and organ damage over time. Abnormally low hepcidin levels caused by genetic mutations or secondary pathology can be addressed with a hepcidin mimetic to restore iron homeostasis. PTG-300 has been granted Orphan Drug designation in the U.S. and EU and has received Fast Track designation by the FDA for development in the treatment of beta-thalassemia. Myelodysplastic syndromes or hereditary hemochromatosis represent additional opportunities for the development of PTG-300.
About Protagonist Therapeutics, Inc.
Protagonist Therapeutics is a clinical stage biopharmaceutical company that utilizes a proprietary technology platform to discover and develop novel peptide-based drugs to transform existing treatment paradigms for patients with significant unmet medical needs. PTG-300 is an injectable hepcidin mimetic in development for the potential treatment of iron overload anemia and related rare blood diseases including beta-thalassemia and polycythemia vera. PTG-200 is an oral, gut-restricted interleukin-23 receptor specific antagonist peptide in Phase 2 clinical development for the potential treatment of inflammatory bowel disease, with Crohn's disease as the initial indication. The Company has a worldwide license and collaboration agreement with Janssen Biotech for the clinical development of PTG-200. PN-943 is an oral, gut-restricted alpha-4-beta-7 integrin specific antagonist peptide in clinical development for the potential treatment of inflammatory bowel disease, with a Phase 2 ulcerative colitis study expected to commence in the second quarter of 2020.
Protagonist is headquartered in Newark, California. For further information, please visit http://www.protagonist-inc.com.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding our intentions or current expectations concerning, among other things, the potential of PTG-300 as a possible treatment for TD beta-thalassemia, the Company's success at finding appropriate doses of PTG-300 for the treatment of beta-thalassemia, the results of future studies for the treatment of beta-thalassemia, the potential utility of PTG-300 in blood disorders including beta-thalassemia, polycythemia vera, and hereditary hemochromatosis, as well as myelodysplastic syndromes, the Company's ability to fund its clinical trials, results of the Phase 2 TRANSCEND Study,, the potential of PTG-200 and PN-943 as possible treatments for inflammatory bowel disease, the initiation of and enrollment of patients in our clinical trials, the results of clinical trials and the outlook for our other programs. In some cases, you can identify these statements by forward-looking words such as "plan," "will," "expect," "potential," or the negative or plural of these words or similar expressions. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, our ability to develop and commercialize our product candidates, our ability to earn milestone payments under our collaboration agreement with Janssen, our ability to use and expand our programs to build a pipeline of product candidates, our ability to obtain and maintain regulatory approval of our product candidates. Additional information concerning these and other risk factors affecting our business can be found in our periodic filings with the Securities and Exchange Commission, including under the heading "Risk Factors" contained in our Quarterly Report on Form 10-Q for the quarterly period ended September 30, 2019, filed with the Securities and Exchange Commission. Forward-looking statements are not guarantees of future performance, and our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate, may differ materially from the forward-looking statements contained in this press release. Any forward-looking statements that we make in this press release speak only as of the date of this press release. We assume no obligation to update our forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this press release.
 View original content to download multimedia:http://www.prnewswire.com/news-releases/protagonist-therapeutics-announces-preliminary-phase-2-results-with-hepcidin-mimetic-ptg-300-in-the-treatment-of-transfusion-dependent-beta-thalassemia-300967872.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/protagonist-therapeutics-announces-preliminary-phase-2-results-with-hepcidin-mimetic-ptg-300-in-the-treatment-of-transfusion-dependent-beta-thalassemia-300967872.html
SOURCE Protagonist Therapeutics, Inc.