Orthofix Medical Inc. (NASDAQ:OFIX), a global medical device company focused on musculoskeletal healing products, today announced it has received the U.S. Food and Drug Administration (FDA) 510(k) clearance and European CE Mark approval for the JuniOrtho® Plating System. Created specifically for pediatric patients, this innovative fixation system is designed to address the demands of advanced deformity and trauma reconstruction of the lower extremities.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20200720005092/en/
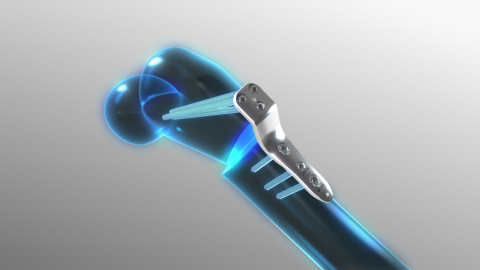
Image of the Orthofix JuniOrtho Plating System (Photo: Business Wire)
“The field of deformity correction is rapidly evolving with multiple technical and technological advances every year,” said Dr. Philip McClure, an orthopedic surgeon at the International Center for Limb Lengthening in Baltimore. “External fixators are an incredibly powerful tool in caring for deformities in children around the world. Improved designs of internal fixation devices have allowed us to provide complimentary options to external fixators to benefit our patients and their families. Our goal is to obtain the best possible outcome so children can enjoy their lives after deformity correction surgery.”
The JuniOrtho Plating System is complemented by a pre-operative planning software option that streamlines the implant selection prior to the surgical procedure. This unique platform enables the surgeon to accurately plan the osteotomy position to visualize the implant in relation to the anatomy. This aids in the selection of the precise size of device to ensure the best fit and optimal positioning for the patient’s body. Specifically developed to be used in combination with the JuniOrtho Plating System, the software is currently available in Europe and planned for release in the U.S. later this year.
“The JuniOrtho Plating System represents our continued commitment to advancing pediatric orthopedics by providing surgeons the devices they need to treat even the smallest of patients,” said Jon Serbousek, President and CEO of Orthofix. “We are excited to now be able to offer surgeons both an internal and external fixation systems to expand our portfolio of pediatric deformity care solutions.”
Offered in a wide range of plate sizes with a variety of lengths, the JuniOrtho Plating System accommodates both locking and non-locking screws corresponding to the plate size. The system is comprised of sterile implants and single-use tools to reduce the risk of contamination and optimize efficiency during the procedure.
The JuniOrtho Plating System is part of the JuniOrtho line of pediatric solutions that includes the TL-HEX™ system, TrueLok Ring Fixation System, eight-Plate Guided Growth System+, and many others. JuniOrtho brings products and resources together to give medical professionals and families alike the best in pediatric orthopedic solutions.
About Orthofix
Orthofix Medical Inc. is a global medical device company focused on musculoskeletal products and therapies. The Company’s mission is to improve patients' lives by providing superior reconstruction and regenerative musculoskeletal solutions to physicians worldwide. Headquartered in Lewisville, Texas, Orthofix’s spine and orthopedic extremities products are distributed in more than 70 countries via the Company's sales representatives and distributors. For more information, please visit www.orthofix.com.
Forward Looking Statements
This communication contains certain forward-looking statements under the Private Securities Litigation Reform Act of 1995. These forward-looking statements, which may include, but are not limited to, statements concerning the estimates, projections, financial condition, results of operations and businesses of Orthofix and its subsidiaries, are based on Orthofix management's current expectations and estimates and involve risks and uncertainties that could cause actual results or outcomes to differ materially from those contemplated by the forward-looking statements.
The forward-looking statements in this release do not constitute guarantees or promises of future performance. Factors that could cause or contribute to such differences may include, but are not limited to, risks relating to: practices of health insurance companies and other third-party payors with respect to reimbursement for our devices and other risks described in the "Risk Factors" section of our 2019 Annual Report on Form 10-K, as well as in other reports that we file in the future. Existing and prospective investors are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. The Company undertakes no obligation to update or revise the information contained in this press release.

View source version on businesswire.com: https://www.businesswire.com/news/home/20200720005092/en/
Copyright Business Wire 2020