ACE-LY-004 Phase II trial results substantiate established efficacy and safety profile of CALQUENCE in mantle cell lymphoma
In the release that dated December 6, 2020, the "Summary of efficacy results" table should appear as follows:
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20201206005037/en/
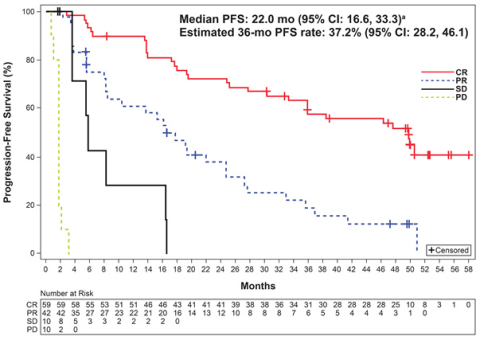
Figure. Progression-free survival1. CR, complete response; OS, overall survival; PD, progressive disease; PFS, progression-free survival; PR, partial response; SD, stable disease (Photo: Business Wire)
Summary of efficacy results 1
Median follow up of 38.1 months (range: 0.3-59.5)
|
Efficacy measure
|
Result (N=124; 95% CI)
|
|
ORR (investigator-assessed PR or better per Lugano classification), %
|
81 (74, 88)
|
|
CR, %
|
48 (39, 57)
|
|
Median DOR, months
|
28.6 (17.5, 39.1)
|
|
Estimated DOR rate at 36 months, %
|
41.9 (31.7, 51.8)
|
|
Median PFS, months
|
22.0 (16.6, 33.3)
|
|
Estimated PFS rate at 36 months, %
|
37.2 (28.2, 46.1)
|
The corrected release reads:
CALQUENCE Shows Long-Term Efficacy and Tolerability at Three Years for Patients With Relapsed or Refractory Mantle Cell Lymphoma
ACE-LY-004 Phase II trial results substantiate established efficacy and safety profile of CALQUENCE in mantle cell lymphoma
Long-term follow-up results from the positive ACE-LY-004 Phase II trial showed patients with relapsed or refractory mantle cell lymphoma (MCL) treated with CALQUENCE ® (acalabrutinib) remained progression free for a median of 22 months, with median overall survival not yet reached at three years of follow-up. The safety and tolerability profile remained consistent. 1 This data was presented at the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition on 6 December 2020.
MCL is typically an aggressive, rare form of non-Hodgkin lymphoma (NHL) that accounts for nearly 6% of all NHL cases and is mostly found in males during their early sixties. 2,3
At a median follow up of 38.1 months (range: 0.3-59.5), 55 patients (44%) either remained on treatment (24 patients) or continued to be followed for survival (31 patients). The safety profile remained largely unchanged from the last analysis at 26 months, with only 14 patients (11%) having discontinued treatment due to adverse events (AEs). 1
Michael L. Wang, MD, Professor, Department of Lymphoma/Myeloma, The University of Texas MD Anderson Cancer Center, and principal investigator of the ACE-LY-004 Phase II trial, said: “Mantle cell lymphoma is an aggressive, difficult-to-treat blood cancer that is typically diagnosed at an advanced stage and often becomes resistant to treatment. This data shows that patients treated with acalabrutinib experienced deep responses over time, while the safety profile remained largely the same, including low rates of Grade 3/4 events, cardiac events and bleeding events, which are important in this patient population.”
José Baselga, Executive Vice President, Oncology R&D, said: “These results add to the mounting evidence that CALQUENCE can provide sustained responses in patients over more than three years. CALQUENCE is an important chemo-free treatment option for relapsed or refractory mantle cell lymphoma and is rapidly being embraced across the clinical and patient community.”
Summary of efficacy results 1
Median follow up of 38.1 months (range: 0.3-59.5)
|
Efficacy measure
|
Result (N=124; 95% CI)
|
|
ORR (investigator-assessed PR or better per Lugano classification), %
|
81 (74, 88)
|
|
CR, %
|
48 (39, 57)
|
|
Median DOR, months
|
28.6 (17.5, 39.1)
|
|
Estimated DOR rate at 36 months, %
|
41.9 (31.7, 51.8)
|
|
Median PFS, months
|
22.0 (16.6, 33.3)
|
|
Estimated PFS rate at 36 months, %
|
37.2 (28.2, 46.1)
|
|
CI, confidence interval; ORR, overall response rate; PR, partial response; CR, complete response; DOR, duration of response; PFS, progression-free survival; DOR was measured in the 101 subjects who achieved a CR or PR
|
Additionally, an exploratory analysis of 30 patients meeting the criteria for minimal residual disease (MRD) evaluation showed six patients (20%) achieved a complete response and undetectable MRD (uMRD) and maintained uMRD at last assessment. 1
AEs in the trial remained largely unchanged with an additional year of follow up. The most frequent AEs of any grade (greater than or equal to 20% of patients) included headache (39%), diarrhea (37%), fatigue (30%), cough (23%), myalgia (22%) and nausea (22%), and were primarily Grade 1/2. Grade 3/4 AEs included neutropenia (11%), anemia (10%) and pneumonia (6%). 1
Overall, 16 patients (13%) had cardiac AEs (11 with prior cardiac risk factors), with three of the 16 cardiac AEs occurring in the last year of follow up (two were Grade 3/4). Overall, six patients (5%) had Grade 3/4 cardiac AEs. One patient had Grade 3/4 hypertension in the last year (total any grade, n=5 [4%]; total Grade 3/4, n=2 [2%]), and five patients had bleeding AEs in the last year (total any grade, n=46 [37%]). Three patients had Grade 3/4 infections in the last year. 1
Initial results from the ACE-LY-004 Phase II trial were presented on 9 December 2017 at the 59th ASH Annual Meeting and Exposition and served as the basis for the first FDA approval of CALQUENCE in adult patients with MCL who have received at least one prior therapy. 4 CALQUENCE is approved for this indication in many other countries. The US MCL indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
INDICATION AND USAGE
CALQUENCE is a Bruton tyrosine kinase (BTK) inhibitor indicated for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy.
This indication is approved under accelerated approval based on overall response rate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
CALQUENCE is also indicated for the treatment of adult patients with chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL).
IMPORTANT SAFETY INFORMATION ABOUT CALQUENCE ® (acalabrutinib) capsules
Serious and Opportunistic Infections
Fatal and serious infections, including opportunistic infections, have occurred in patients with hematologic malignancies treated with CALQUENCE.
Serious or Grade 3 or higher infections (bacterial, viral, or fungal) occurred in 19% of 1029 patients exposed to CALQUENCE in clinical trials, most often due to respiratory tract infections (11% of all patients, including pneumonia in 6%). These infections predominantly occurred in the absence of Grade 3 or 4 neutropenia, with neutropenic infection reported in 1.9% of all patients. Opportunistic infections in recipients of CALQUENCE have included, but are not limited to, hepatitis B virus reactivation, fungal pneumonia, Pneumocystis jiroveci pneumonia, Epstein-Barr virus reactivation, cytomegalovirus, and progressive multifocal leukoencephalopathy (PML). Consider prophylaxis in patients who are at increased risk for opportunistic infections. Monitor patients for signs and symptoms of infection and treat promptly.
Hemorrhage
Fatal and serious hemorrhagic events have occurred in patients with hematologic malignancies treated with CALQUENCE. Major hemorrhage (serious or Grade 3 or higher bleeding or any central nervous system bleeding) occurred in 3.0% of patients, with fatal hemorrhage occurring in 0.1% of 1029 patients exposed to CALQUENCE in clinical trials. Bleeding events of any grade, excluding bruising and petechiae, occurred in 22% of patients.
Use of antithrombotic agents concomitantly with CALQUENCE may further increase the risk of hemorrhage. In clinical trials, major hemorrhage occurred in 2.7% of patients taking CALQUENCE without antithrombotic agents and 3.6% of patients taking CALQUENCE with antithrombotic agents. Consider the risks and benefits of antithrombotic agents when co-administered with CALQUENCE. Monitor patients for signs of bleeding.
Consider the benefit-risk of withholding CALQUENCE for 3-7 days pre- and post-surgery depending upon the type of surgery and the risk of bleeding.
Cytopenias
Grade 3 or 4 cytopenias, including neutropenia (23%), anemia (8%), thrombocytopenia (7%), and lymphopenia (7%), developed in patients with hematologic malignancies treated with CALQUENCE. Grade 4 neutropenia developed in 12% of patients. Monitor complete blood counts regularly during treatment. Interrupt treatment, reduce the dose, or discontinue treatment as warranted.
Second Primary Malignancies
Second primary malignancies, including skin cancers and other solid tumors, occurred in 12% of 1029 patients exposed to CALQUENCE in clinical trials. The most frequent second primary malignancy was skin cancer, reported in 6% of patients. Monitor patients for skin cancers and advise protection from sun exposure.
Atrial Fibrillation and Flutter
Grade 3 atrial fibrillation or flutter occurred in 1.1% of 1029 patients treated with CALQUENCE, with all grades of atrial fibrillation or flutter reported in 4.1% of all patients. The risk may be increased in patients with cardiac risk factors, hypertension, previous arrhythmias, and acute infection. Monitor for symptoms of arrhythmia (e.g., palpitations, dizziness, syncope, dyspnea) and manage as appropriate.
ADVERSE REACTIONS
The most common adverse reactions (≥ 20%) of any grade in patients with relapsed or refractory MCL were anemia,* thrombocytopenia,* headache (39%), neutropenia,* diarrhea (31%), fatigue (28%), myalgia (21%), and bruising (21%). The most common Grade ≥ 3 non-hematological adverse reaction (reported in at least 2% of patients) was diarrhea (3.2%).
*Treatment-emergent decreases (all grades) of hemoglobin (46%), platelets (44%), and neutrophils (36%) were based on laboratory measurements and adverse reactions.
Dose reductions or discontinuations due to any adverse reaction were reported in 1.6% and 6.5% of patients, respectively. Increases in creatinine 1.5 to 3 times the upper limit of normal occurred in 4.8% of patients.
The most common adverse reactions (≥ 30%) of any grade in patients with CLL were anemia,* neutropenia,* thrombocytopenia,* headache, upper respiratory tract infection, and diarrhea.
*Treatment-emergent decreases (all grades) of hemoglobin, platelets, and neutrophils were based on laboratory measurements and adverse reactions.
In patients with previously untreated CLL exposed to CALQUENCE, fatal adverse reactions that occurred in the absence of disease progression and with onset within 30 days of the last study treatment were reported in 2% for each treatment arm, most often from infection. Serious adverse reactions were reported in 39% of patients in the CALQUENCE plus obinutuzumab arm and 32% in the CALQUENCE monotherapy arm, most often due to events of pneumonia (7% and 2.8%, respectively).
Adverse reactions led to CALQUENCE dose reduction in 7% and 4% of patients in the CALQUENCE plus obinutuzumab arm (N=178) and CALQUENCE monotherapy arm (N=179), respectively. Adverse events led to discontinuation in 11% and 10% of patients, respectively. Increases in creatinine 1.5 to 3 times the upper limit of normal occurred in 3.9% and 2.8% of patients in the CALQUENCE combination arm and monotherapy arm, respectively.
In patients with relapsed/refractory CLL exposed to CALQUENCE, serious adverse reactions occurred in 29% of patients. Serious adverse reactions in > 5% of patients who received CALQUENCE included lower respiratory tract infection (6%). Fatal adverse reactions within 30 days of the last dose of CALQUENCE occurred in 2.6% of patients, including from second primary malignancies and infection.
Adverse reactions led to CALQUENCE dose reduction in 3.9% of patients (N=154), dose interruptions in 34% of patients, most often due to respiratory tract infections followed by neutropenia, and discontinuation in 10% of patients, most frequently due to second primary malignancies followed by infection. Increases in creatinine 1.5 to 3 times the upper limit of normal occurred in 1.3% of patients who received CALQUENCE.
DRUG INTERACTIONS
Strong CYP3A Inhibitors: Avoid co-administration with a strong CYP3A inhibitor. If a strong CYP3A inhibitor will be used short-term, interrupt CALQUENCE.
Moderate CYP3A Inhibitors: When CALQUENCE is co-administered with a moderate CYP3A inhibitor, reduce CALQUENCE dose to 100 mg once daily.
Strong CYP3A Inducers: Avoid co-administration with a strong CYP3A inducer. If a strong CYP3A inducer cannot be avoided, increase the CALQUENCE dose to 200 mg approximately every 12 hours.
Gastric Acid Reducing Agents: If treatment with a gastric acid reducing agent is required, consider using an H2-receptor antagonist or an antacid. Take CALQUENCE 2 hours before taking an H2-receptor antagonist. Separate dosing with an antacid by at least 2 hours.
Avoid co-administration with proton pump inhibitors. Due to the long-lasting effect of proton pump inhibitors, separation of doses may not eliminate the interaction with CALQUENCE.
SPECIFIC POPULATIONS
Based on findings in animals, CALQUENCE may cause fetal harm and dystocia when administered to a pregnant woman. There are no available data in pregnant women to inform the drug-associated risk. Advise pregnant women of the potential risk to a fetus.
Pregnancy testing is recommended for females of reproductive potential prior to initiating CALQUENCE therapy. Advise female patients of reproductive potential to use effective contraception during treatment with CALQUENCE and for at least 1 week following the last dose of CALQUENCE.
It is not known if CALQUENCE is present in human milk. Advise lactating women not to breastfeed while taking CALQUENCE and for at least 2 weeks after the final dose. Avoid administration of CALQUENCE in patients with severe hepatic impairment. Dose modifications are not required for patients with mild or moderate hepatic impairment.
Please see full Prescribing Information , including Patient Information .
Mantle cell lymphoma
Mantle cell lymphoma (MCL) is an uncommon type of B-cell non-Hodgkin lymphoma. 5 MCL comprises 3% to 6% of non-Hodgkin lymphomas, with an annual incidence of 0.5 per 100,000 population in Western countries; in the US, it was estimated that approximately 3,300 new cases of MCL were diagnosed in 2016. 5,6 The median age at diagnosis is 68 years, with MCL occurring more often in men than women. While MCL patients initially respond to treatment, there is a high relapse rate. 5
ACE-LY-004
ACE-LY-004 is an open-label, single-arm Phase II clinical trial evaluating CALQUENCE in adult patients with relapsed or refractory MCL. 7 Adults with MCL and ECOG PS ≤2 who had relapsed or were refractory to 1-5 prior therapies, had no prior BTK/BCL-2 inhibitor exposure, and did not require warfarin/vitamin K antagonists, received oral CALQUENCE 100mg twice-daily until progressive disease or toxicity. Overall response rate (investigator-assessed partial response or better per Lugano classification), duration of response, progression-free survival, overall survival, and safety were assessed. Minimal residual disease was analyzed in formalin-fixed, paraffin-embedded samples and peripheral blood by next-generation sequencing (5x10 -6 ) in patients with available paired samples. 1
CALQUENCE
CALQUENCE (acalabrutinib) is a next-generation, selective inhibitor of BTK. CALQUENCE binds covalently to BTK, thereby inhibiting its activity. 4,8 In B-cells, BTK signaling results in activation of pathways necessary for B-cell proliferation, trafficking, chemotaxis, and adhesion. 4
CALQUENCE is approved for the treatment of CLL and SLL in the US and is approved for CLL in the EU and several other countries worldwide. CALQUENCE is also approved for the treatment of adult patients with MCL who have received at least one prior therapy in the US and several other countries. CALQUENCE is not currently approved for the treatment of MCL in Europe.
As part of an extensive clinical development program, AstraZeneca and Acerta Pharma are currently evaluating CALQUENCE in more than 20 company-sponsored clinical trials. CALQUENCE is being developed for the treatment of multiple B-cell blood cancers including CLL, MCL, diffuse large B-cell lymphoma, Waldenström’s macroglobulinemia, follicular lymphoma, and other hematologic malignancies.
AstraZeneca in hematology
Leveraging its strength in oncology, AstraZeneca has established hematology as one of four key oncology disease areas of focus. The Company’s hematology franchise includes two medicines approved by the US Food and Drug Administration and a robust global development program for a broad portfolio of potential blood cancer treatments. Acerta Pharma serves as AstraZeneca’s hematology research and development arm. AstraZeneca partners with like-minded science-led companies to advance the discovery and development of therapies to address unmet need.
AstraZeneca in oncology
AstraZeneca has a deep-rooted heritage in oncology and offers a quickly growing portfolio of new medicines that has the potential to transform patients' lives and the Company's future. With seven new medicines launched between 2014 and 2020, and a broad pipeline of small molecules and biologics in development, the Company is committed to advance oncology as a key growth driver for AstraZeneca focused on lung, ovarian, breast and blood cancers.
By harnessing the power of six scientific platforms - Immuno-Oncology, Tumor Drivers and Resistance, DNA Damage Response, Antibody Drug Conjugates, Epigenetics, and Cell Therapies - and by championing the development of personalized combinations, AstraZeneca has the vision to redefine cancer treatment and one day eliminate cancer as a cause of death.
About AstraZeneca
AstraZeneca is a global, science-led biopharmaceutical company that focuses on the discovery, development and commercialization of prescription medicines, primarily for the treatment of diseases in three therapy areas - Oncology, Cardiovascular, Renal & Metabolism, and Respiratory & Immunology. AstraZeneca operates in over 100 countries and its innovative medicines are used by millions of patients worldwide. For more information, please visit astrazeneca-us.com and follow us on Twitter @AstraZenecaUS .
References
- Wang M, et al. Acalabrutinib Monotherapy in Patients With Relapsed/Refractory Mantle Cell Lymphoma: Long-Term Efficacy and Safety Results From a Phase 2 Study. Abstract #2040 at the 62nd American Society of Hematology (ASH) Annual Meeting and Exposition.
- O’Connor OA and Vose JM. Mantle Cell Lymphoma: Getting the Facts. Lymphoma Research Foundation. Available at: https://lymphoma.org/aboutlymphoma/nhl/mcl/ .
- American Cancer Society. Non Hodgkin Lymphoma. Available at: https://www.cancer.org/cancer/non-hodgkin-lymphoma.html .
- CALQUENCE (acalabrutinib) [prescribing information]. Wilmington, DE; AstraZeneca Pharmaceuticals LP; 2019.
- Cheah CY, et al. Mantle cell lymphoma. J Clin Oncol . 10 Apr 2016;34(11):1256-1269. Published online ahead of print on 11 Jan 2016.
- Teras LR, et al. 2016 US Lymphoid Malignancy Statistics by World Health Organization Subtypes. Ca Cancer J Clin . 2016;66(6):443-459.
- ClinicalTrials.gov. An Open-label, Phase 2 Study of ACP-196 (Acalabrutinib) in Subjects With Mantle Cell Lymphoma. Available at: https://www.clinicaltrials.gov/ct2/show/NCT02213926 .
- Wu J, et al. Acalabrutinib (ACP-196): a selective second generation BTK inhibitor. J Hematol Oncol . 2016;9(21).

View source version on businesswire.com: https://www.businesswire.com/news/home/20201206005037/en/
Media Inquiries
Michele Meixell +1 302 885 2677
Brendan McEvoy +1 302 885 2677