YARDLEY, Pa. , June 2, 2021 /PRNewswire/ -- Optinose (NASDAQ:OPTN), a global specialty pharmaceutical company focused on serving the needs of patients cared for by ear, nose and throat (ENT) and allergy specialists, today announced that the publication of a new stepped-care treatment algorithm in the International Forum of Allergy & Rhinology , " Multidisciplinary consensus on a stepwise treatment algorithm for management of chronic rhinosinusitis with nasal polyps ," recommends Exhalation Delivery System-fluticasone (EDS-FLU) for the treatment of patients with nasal polyps. It also recommends EDS-FLU as a step in between initial care with standard intranasal steroids and before escalation of care with surgery or biologic medicines.
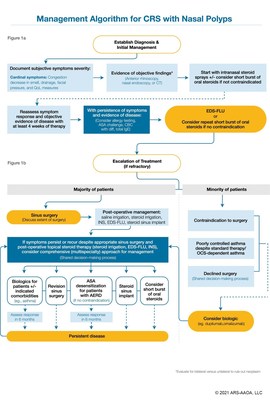
The algorithm was informed by evidence-based, peer-reviewed data supporting the use of therapeutic and interventional treatments to arrive at the recommended stepwise care paradigm. The growing number of medical and surgical treatment options available for patients with nasal polyps can cause confusion among providers regarding the optimal sequence for those treatment modalities. The algorithm seeks to lend order to treatment and serve as a basis for improving quality of care. The publication discusses considerations in a pathway for care that incorporates several new treatments approved in recent years into a logical stepwise escalation of medical care, including but not limited to standard nasal steroid sprays, EDS-FLU, surgery, implants and biologics.
"Over 85% of patients suffering from chronic rhinosinusitis with nasal polyps are frustrated with the lack of symptom relief they get with standard nasal steroids," 1 said Ramy Mahmoud , M.D., MPH, President of Optinose. "This is unsurprising since inflammation high and deep in the nasal cavity lies at the root of the disease and can be difficult to reach with standard nasal steroid sprays that patients typically try first." 2,3
XHANCE was approved by the U.S. Food and Drug Administration in 2017 for the treatment of nasal polyps in patients 18 years of age and older and is the only medication available that uses the EDS to deliver an anti-inflammatory medicine high and deep to the source of the problem.
"Our goal is to share an emerging logical stepped-care guidance similar to what is used in other disease areas such as asthma in which treatment is escalated stepwise in attempt to achieve a full response," said Dr. Joseph Han , Chief for the Division of Rhinology – Endoscopic Sinus and Skull Base Surgery and Chief for the Division of Allergy at Eastern Virginia Medical School and first author of the consensus paper. "Stepped-care guidelines in medicine can serve as a basis for reducing confusion and improving quality of care. We hope that physicians, as well as payers, will find this new approach useful in guiding individual patient-based decision making."
In addition to Dr. Han, current President of the American Rhinologic Society, the expert panel that developed the consensus paper is composed of otolaryngologists Christine Franzese , M.D., Kent Lam , M.D., Andrew P. Lane , M.D., Stella Lee , M.D., James Palmer , M.D., Zachary Soler , M.D., and Jivianne Lee, M.D., and allergists John V. Bosso , M.D., Seong H. Cho , M.D., and Anju Peters , M.D.
About Nasal Polyps
Nasal polyps are soft, noncancerous (or benign) protrusions high in the lining of the nasal passages or sinuses. Nasal polyps generally develop as part of the inflammatory process of chronic rhinosinusitis (CRS) and can worsen inflammatory blockage of normal ventilation and drainage from the sinuses. CRS is a chronic nasal inflammatory disease that may affect as many as 30 million adults in the United States . 4 Consideration should be given to the possibility of nasal polyps in patients with symptoms of chronic rhinosinusitis who are insufficiently responsive to traditional intranasal steroid sprays. It is estimated that 10 million patients suffer from symptoms of nasal polyps in the U.S. alone, leading to approximately $5.7 billion in direct costs annually. 5
Treatments for nasal polyps include, among others, standard topical intranasal steroid sprays, XHANCE, short bursts of oral steroids, surgery, implants and biologics.
About Optinose
Optinose is a global specialty pharmaceutical company focused on serving the needs of patients cared for by ear, nose and throat (ENT) and allergy specialists. To learn more, please visit www.optinose.com or follow us on Twitter and LinkedIn .
About XHANCE ®
XHANCE uses an Optinose Exhalation Delivery System (EDS™) designed to deliver a topically acting anti-inflammatory corticosteroid to the high and deep regions of the nasal cavity where nasal polyps originate. XHANCE was approved for the treatment of nasal polyps in patients 18 years of age or older by the U.S. Food and Drug Administration in September 2017.
Important Safety Information
CONTRAINDICATIONS: Hypersensitivity to any ingredient in XHANCE.
WARNINGS AND PRECAUTIONS:
- Local Nasal Effects: epistaxis, erosion, ulceration, septal perforation, Candida albicans infection, and impaired wound healing. Monitor patients periodically for signs of possible changes on the nasal mucosa. Avoid use in patients with recent nasal ulcerations, nasal surgery, or nasal trauma.
- Close monitoring for glaucoma and cataracts is warranted.
- Hypersensitivity reactions (e.g., anaphylaxis, angioedema, urticaria, contact dermatitis, rash, hypotension, and bronchospasm) have been reported after administration of fluticasone propionate. Discontinue XHANCE if such reactions occur.
- Immunosuppression: potential increased susceptibility to or worsening of infections (e.g., existing tuberculosis; fungal, bacterial, viral, or parasitic infection; ocular herpes simplex). Use with caution in patients with these infections. More serious or even fatal course of chickenpox or measles can occur in susceptible patients.
- Hypercorticism and adrenal suppression may occur with very high dosages or at the regular dosage in susceptible individuals. If such changes occur, discontinue XHANCE slowly.
- Patients with major risk factors for decreased bone mineral content should be monitored and treated with established standards of care.
ADVERSE REACTIONS: The most common adverse reactions (incidence ≥ 3%) are epistaxis, nasal septal ulceration, nasopharyngitis, nasal mucosal erythema, nasal mucosal ulcerations, nasal congestion, acute sinusitis, nasal septal erythema, headache, and pharyngitis.
DRUG INTERACTIONS: Strong cytochrome P450 3A4 inhibitors (e.g., ritonavir, ketoconazole): Use not recommended. May increase risk of systemic corticosteroid effects.
USE IN SPECIFIC POPULATIONS: Hepatic impairment. Monitor patients for signs of increased drug exposure.
Please see full Prescribing Information .
Media Contact:
Jonathan Neely
(267) 521-0531
Jonathan.Neely@optinose.com
Melissa Katz
(215) 514-0957
Mkatz@stonyhillcomms.com
1 Palmer et al. Allergy and Asthma Proc. 2019;40(1):48-56.
2 Sindwani, R., Han, J. K., Soteres, D. F., Messina , J. C., Carothers, J. L., Mahmoud, R. A., & Djupesland, P. G. (2019). NAVIGATE I: Randomized, Placebo-Controlled, Double-Blind Trial of the Exhalation Delivery System With Fluticasone for Chronic Rhinosinusitis With Nasal Polyps. American journal of rhinology & allergy , 33(1), 69–82. https://doi.org/10.1177/1945892418810281
3 Leopold, D. A., Elkayam, D., Messina , J. C., Kosik-Gonzalez, C., Djupesland, P. G., & Mahmoud, R. A. (2019). NAVIGATE II: Randomized, double-blind trial of the exhalation delivery system with fluticasone for nasal polyposis. The Journal of allergy and clinical immunology , 143(1), 126–134.e5. https://doi.org/10.1016/j.jaci.2018.06.010
4 Pearlman AN, Chandra RK, Chang D, et al. Relationships between severity of chronic rhinosinusitis and nasal polyposis, asthma, and atopy. Am J Rhinol Allergy. 2009;23(2):145-148.
5 Bhattacharyya et al. Laryngoscope. 2019;129(9):1969-1975.

 View original content to download multimedia: http://www.prnewswire.com/news-releases/experts-issue-new-treatment-algorithm-for-chronic-rhinosinusitis-with-nasal-polyps-that-highlights-role-of-xhance-in-stepwise-care-301303557.html
View original content to download multimedia: http://www.prnewswire.com/news-releases/experts-issue-new-treatment-algorithm-for-chronic-rhinosinusitis-with-nasal-polyps-that-highlights-role-of-xhance-in-stepwise-care-301303557.html
SOURCE Optinose