PharmAla's GMP LaNeo™ MDMA has completed all release testing, is ready for export
VANCOUVER, BC, Aug. 17, 2022 /CNW/ - PharmAla Biotech (CSE: MDMA) (the 'Company') has achieved a historic milestone: it is currently the only publicly-traded company to have produced GMP MDMA at scale. The company believes that it is currently the only source for commercially-available GMP MDMA other than the Multidisciplinary Association for Psychedelic Studies (MAPS). With the release of this large-scale batch of LaNeo™ MDMA Active Pharmaceutical Ingredient (API), PharmAla's GMP value-chain stands ready to support its clinical trial customers and partners globally.
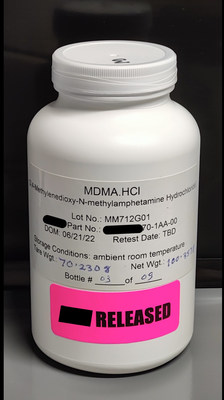
"After a year of intensive work, we're thrilled to be able to announce this historic milestone for the company. As the only GMP MDMA manufacturer in the Americas, we're excited to do our part to alleviate the global backlog of clinical-grade MDMA," said Nick Kadysh, CEO of PharmAla Biotech, and Chair of the Board of Psychedelics Canada. "As research into MDMA dramatically increases and a number of countries begin to move to more permissive 'expanded access' regimes, PharmAla Biotech is perfectly positioned to continue to support scientific discovery by providing the clinical-grade drug product our research partners need."
PharmAla's LaNeo™ MDMA API is scheduled to be formulated into a number of dosage forms, over the coming number of months, with the first being a 40mg capsule. It can also be compounded directly by pharmacists, where permitted by local regulations.
"Access to GMP research materials is often a rate liming step in clinical research; but now with greater access, the science will advance much faster, as will the potential for improving patient care" said Dr. Leah Mayo, incoming Parker Psychedelic Research Chair, University of Calgary.
"Over the past 6 months, we've heard endlessly about the global supply chain crisis in Psychedelic molecules. It's gratifying to now be in a position to assist our partners worldwide by shipping Drug Substance and Product to them, allowing them to get on with their vital research," said Dr. Shane Morris, PharmAla's COO. "We're confident that, once researchers initiate clinical work with PharmAla's LaNeo™ MDMA, it will help drive interest in clinical trials for this impressive drug that several patient groups are urgently requesting."
For more information, please visit www.PharmAla.ca, where you can sign up to receive regular new updates.
About PharmAla
PharmAla Biotech Holdings Inc. (CSE: MDMA) is a biotechnology company focused on the research, development, and manufacturing of MDXX class molecules, including MDMA. PharmAla was founded with a dual focus: alleviating the global backlog of generic, clinical-grade MDMA to enable clinical trials, and to develop novel drugs in the same class. PharmAla is a "regulatory first" organization, formed under the principle that true success in the psychedelics industry will only be achieved through excellent relationships with regulators. Our team of dedicated professionals includes regulatory experts, scientists, and biomanufacturing professionals. PharmAla has built what it believes to be North America's first cGMP MDMA value chain, encompassing GMP manufacturing of Active Pharmaceutical Ingredient (API), and drug product formulation. PharmAla's research and development unit has also begun preclinical research into two patented Novel Chemical Entities (NCEs) based on MDXX class molecules, with proof-of-concept research currently ongoing at the University of Arkansas School for Medical Sciences in the United States and at InterVivo Solutions in Canada. For more information, visit www.PharmAla.ca.
Neither the Canadian Securities Exchange nor its Regulation Services Provider have reviewed or accept responsibility for the adequacy or accuracy of this release.
Cautionary Statement
This press release contains 'forward-looking information' within the meaning of applicable Canadian securities legislation. These statements relate to future events or future performance. The use of any of the words "could", "intend", "expect", "believe", "will", "projected", "estimated" and similar expressions and statements relating to matters that are not historical facts are intended to identify forward-looking information and are based on PharmAla's current belief or assumptions as to the outcome and timing of such future events. Forward-looking information is based on reasonable assumptions that have been made by PharmAla at the date of the information and is subject to known and unknown risks, uncertainties, and other factors that may cause actual results or events to differ materially from those anticipated in the forward-looking information. The forward-looking information contained in this press release is made as of the date hereof, and PharmAla is not obligated to update or revise any forward-looking information, whether as a result of new information, future events or otherwise, except as required by applicable securities laws. Factors that could cause actual results to differ materially from those anticipated in these forward-looking statements are described under the caption "Risk Factors" in PharmAla's management's discussion and analysis which is available on PharmAla's profile at www.sedar.com.
This news release does not constitute an offer to sell or the solicitation of an offer to buy, and shall not constitute an offer, solicitation or sale in any state, province, territory or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state, province, territory or jurisdiction
SOURCE PharmAla Biotech Inc.

 View original content to download multimedia: http://www.newswire.ca/en/releases/archive/August2022/17/c4496.html
View original content to download multimedia: http://www.newswire.ca/en/releases/archive/August2022/17/c4496.html