Orthofix Medical Inc. (NASDAQ:OFIX), a leading global spine and orthopedics company, today announced the full commercial launch of, and first patient cases involving, the Mariner®Deformity Pedicle Screw System.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230126005340/en/
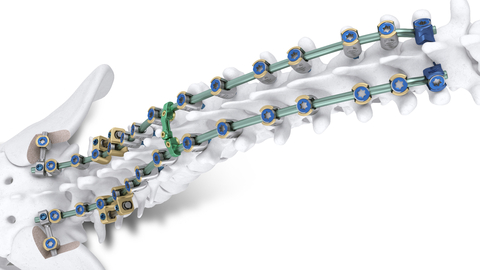
Image of the Mariner Deformity Pedicle Screw System for complex adult deformity spine cases. (Photo: Business Wire)
The first product launched since the merger of Orthofix and SeaSpine earlier this month, the Mariner Deformity Pedicle Screw System developed by SeaSpine is built upon the strength and versatility of the foundational Mariner Pedicle Screw System to address the unique clinical requirements of complex adult deformity spine cases. The full commercial launch adds advanced reduction and correction instrumentation, specialized implant technologies, and robust osteotomy tools to deliver efficient and powerful surgical intervention.
“The Mariner Deformity System represents an innovative collaboration between surgical minds and a cutting-edge product development team,” stated Dennis Cirino, SeaSpine’s Senior Vice President, Global Spinal Systems. “Combined with our market-leading biologics and 7D FLASH™ navigation with machine vision accuracy and segmental registration, this system is designed to deliver a full solution that meets clinical needs and advances the modular platform of the Mariner family.”
The Mariner Deformity System incorporates the strength of Mariner’s modular technology to provide a comprehensive implant offering while reducing the number of surgical trays typically associated with deformity surgery. This reduced physical footprint is intended to result in improved workflow, limited operating room clutter, and reduced sterile processing costs to the hospital. Furthermore, this system expands the differentiated capabilities of the novel “gimbal” technology incorporated into the Mariner family of products to enable more efficient placement of complex instrumentation.
“The Mariner Deformity System has benefitted from both ergonomic refinement and strategic product differentiation,” stated Pawel Jankowski, M.D., neurosurgeon at Hoag Memorial Hospital Presbyterian in Newport Beach, California. “The team has done a tremendous job in creating an inspired, comprehensive complex adult correction platform.”
About the Combined Company
The newly merged Orthofix-SeaSpine organization is a leading global spine and orthopedics company with a comprehensive portfolio of biologics, innovative spinal hardware, bone growth therapies, specialized orthopedic solutions and a leading surgical navigation system. Its products are distributed in approximately 68 countries worldwide.
The Company is headquartered in Lewisville, Texas and has primary offices in Carlsbad, CA, with a focus on spine and biologics product innovation and surgeon education, and Verona, Italy, with an emphasis on product innovation, production, and medical education for orthopedics. The combined company’s global R&D, commercial and manufacturing footprint also includes facilities and offices in Irvine, CA, Toronto, Canada, Sunnyvale, CA, Wayne, PA, Olive Branch, MS, Maidenhead, UK, Munich, Germany, Paris, France and Sao Paulo, Brazil.
Forward-Looking Statements
This news release includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended, and Section 27A of the Securities Act of 1933, as amended. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “projects,” “intends,” “predicts,” “potential,” “continue” or other comparable terminology. Orthofix cautions you that statements included in this news release that are not a description of historical facts are forward-looking statements that are based on the Company’s current expectations and assumptions. Forward-looking statements in this news release include, but are not limited to, statements related to: the objectives of product design and the ability of the underlying products to achieve design objectives, including the ability of the Mariner Deformity System to deliver a full solution that meets clinical needs, advance the modular platform of the Mariner family, and improve workflow, limit operating room clutter, reduce sterile processing costs, and enable more efficient placement of complex instrumentation. Each forward-looking statement contained in this news release is subject to risks and uncertainties that could cause actual results to differ materially from those expressed or implied by such statement. Applicable risks and uncertainties include, among others: the ability of newly launched products to perform as designed and intended and to meet the needs of surgeons and patients, including as a result of the lack of clinical validation of products in limited commercial (or “alpha”) launch; the failure to achieve the anticipated benefits of new products; and the risks identified under the heading “Risk Factors” in Orthofix Medical Inc.’s Annual Report on Form 10-K for the fiscal year ended December 31, 2021, which was filed with the Securities and Exchange Commission (SEC) on February 25, 2022, and SeaSpine Holdings Corporation’s Annual Report on Form 10-K for the fiscal year ended December 31, 2021, filed with the SEC on March 15, 2022, as well as both companies’ subsequent Quarterly Reports on Form 10-Q and other information filed by each company with the SEC. The Company’s public filings with the Securities and Exchange Commission are available at www.sec.gov. You are cautioned not to place undue reliance on forward-looking statements, which speak only as of the date when made. Orthofix does not intend to revise or update any forward-looking statement set forth in this news release to reflect events or circumstances arising after the date hereof, except as may be required by law.

View source version on businesswire.com: https://www.businesswire.com/news/home/20230126005340/en/