Positive results from the Phase 2b FRONTIER 1 study of JNJ-2113 (formerly PN-235) in moderate-to-severe psoriasis, paving a path forward for initiation of a Phase 3 pivotal study
Highly statistically significant results from the randomized withdrawal portion of the REVIVE study of rusfertide in polycythemia vera, achieving its primary endpoint of proportion of responders on rusfertide versus placebo (p=0.0003)
NEWARK, CA / ACCESSWIRE / March 15, 2023 / Protagonist Therapeutics (Nasdaq:PTGX) ("Protagonist" or "the Company") today reported financial results for the fourth quarter and full year ended December 31, 2022 and provided a corporate update.
"The positive FRONTIER 1 JNJ-2113 (PN-235) data recently reported represents a scientific breakthrough with the potential to transform the treatment landscape for moderate-to-severe plaque psoriasis with oral targeted therapy. We look forward to in-depth presentations of JNJ-2113 pre-clinical and Phase 1 and Phase 2 clinical studies at medical conferences beginning in the second quarter of 2023," said Dinesh V. Patel, Ph.D., President and Chief Executive Officer of Protagonist. "In addition, we are extremely pleased to have announced this morning the positive results from the randomized withdrawal part of the REVIVE study of rusfertide in polycythemia vera (PV). The study met its primary endpoint, with a statistically significant higher number of responders on rusfertide versus placebo (p=0.0003). Improvements in symptoms were observed, and rusfertide was generally well tolerated in the overall study."
Dr. Patel continued, "These positive results from the Phase 2 FRONTIER 1 and REVIVE studies, of JNJ-2113 and rusfertide, respectively, are testaments to the promise of our proprietary platform as well as the expertise, experience and commitment of the Protagonist team. We are focused on enrolling patients globally in the Phase 3 VERIFY study of rusfertide in PV, and we are diligently preparing for the pre-commercial launch-related activities for rusfertide.
"Meanwhile, our discovery engine remains prolific. We are evaluating pre-clinical compounds with the potential to have impact in areas of high unmet medical need, and we are specifically advancing efforts in the oral hepcidin mimetic program. With two assets in late-stage development, Protagonist is now closer than ever to bringing transformative new therapies to patients. As an industry leader in the discovery and development of peptide therapeutics, we hold a unique set of capabilities that position our Company for value creation over the long run," Patel said.
Fourth Quarter 2022 Recent Developments and Upcoming Milestones
Rusfertide: Subcutaneous Injectable Hepcidin Mimetic for Polycythemia Vera (PV) and Other Blood Disorders
- The double-blind, placebo-controlled, 12-week randomized withdrawal portion of the REVIVE study met its primary endpoint, phlebotomy eligibility, with subjects randomized to rusfertide demonstrating a highly statistically significant (p=0.0003) proportion of responders compared to placebo. A study subject was defined as a responder if the subject completed 12 weeks of double-blind treatment while maintaining hematocrit control without phlebotomy eligibility and without phlebotomy. During the 12 weeks of the blinded randomized withdrawal, only 2 of 26 subjects on rusfertide were phlebotomized, keeping 92.3% of patients phlebotomy free in the treatment arm.
- In patients with moderate or severe Myeloproliferative Neoplasm-Symptom Assessment Form (MPN-SAF) symptom scores at baseline, the change from baseline was statistically significant in fatigue, problems with concentration, inactivity and itching during the 28-week open label Part 1 of the study. Meaningful comparison of symptom assessments in Part 2 are not possible since a majority of subjects randomized to placebo discontinued prior to the 12-week assessment of MPN-SAF symptoms.
- Rusfertide was well tolerated, with localized injection site reactions comprising the majority of reported adverse events. No new safety signals were observed in these data, following presentation of safety data from the REVIVE study at the American Society of Hematology (ASH) December 2022.
- For PV, Rusfertide has received FDA and EU orphan designation and FDA fast track designation.
JNJ-2113 (formerly PN-235): Oral IL-23 Receptor Antagonist
- The Company announced positive topline results from its collaboration with Janssen Biotech, Inc., one of the Janssen Pharmaceutical Companies of Johnson & Johnson, in the FRONTIER 1 Phase 2b clinical trial evaluating the oral Interleukin-23 receptor (IL-23R) antagonist peptide JNJ-2113 in patients with moderate-to-severe plaque psoriasis.
- Data from the 255-patient study showed that JNJ-2113 achieved the study's primary efficacy endpoint, with a statistically significant greater proportion of patients who received JNJ-2113 achieving PASI-75 (a 75% improvement in skin lesions as measured by the Psoriasis Area and Severity Index) responses compared to placebo at Week 16 in all five treatment groups. A clear dose response was observed across an eight-fold dose range.
- All treatments were well tolerated, with no meaningful difference in frequency of adverse events across treatment groups versus placebo.
- Data from various pre-clinical and clinical studies on JNJ-2113 will be presented at medical conferences beginning in the second quarter of 2023.
- Phase 1 and preclinical data of JNJ-2113 will be presented on May 12, 2023 at the International Societies for Investigative Dermatology Meeting 2023. More information on this presentation, entitled, "First-in-class oral peptide systemically targeting the IL-23 pathway," is available on the ISID 2023 website at isid2023.org.
Fourth Quarter 2022 Financial Results:
- Cash, Cash Equivalents and Marketable Securities: Cash, cash equivalents and marketable securities as of December 31, 2022 were $237.4 million. The Company expects current cash, cash equivalents and marketable securities to be sufficient to fund its planned operating and capital expenditures through the end of 2024.
- License and Collaboration Revenue: License and collaboration revenue for the fourth quarter and full year 2022 were zero and $26.6 million, respectively, as compared to $8.6 million and $27.4 million for the same periods of 2021. The decreases in revenue from prior year periods were primarily due to a decrease in the level of services the Company provided under the Janssen license and collaboration agreement. The Company completed its performance obligation pursuant to the collaboration as of June 30, 2022.
- Research and Development ("R&D") Expenses: R&D expenses for the fourth quarter and full year 2022 were $29.9 million and $126.2 million respectively, as compared to $38.4 million and $126.0 million, respectively, for the same periods of 2021. The decrease in R&D expenses from prior year quarter was primarily due to lower PN-943 expenses related to the suspension of further program expenditures, a decrease in costs related to the completion of JNJ-2113 and PN-232 Phase 1 trials, and a decrease in research and discovery program expenses.
- General and Administrative ("G&A") Expenses: G&A expenses for the fourth quarter and full year 2022 were $6.6 million and $31.7 million, respectively, as compared to $7.3 million and $27.2 million for the same periods of 2021. The decrease in G&A expenses from prior year quarter was primarily due to a decrease in personnel related expenses during the three months ended December 31, 2022. The increase in G&A expenses from prior year was primarily due to increases in personnel related expenses, including stock-based compensation expense, and other expenses to support Company growth.
- Net Loss: Net loss was $34.2 million, or $0.69 per share, for the fourth quarter of 2022 as compared to a net loss of $36.9 million, or $0.77 per share, for the fourth quarter of 2021. Net loss was $127.4 million, or $2.60 per share, for the full year 2022, as compared to a net loss of $125.6 million, or $2.71, for the full year 2021.
PROTAGONIST THERAPEUTICS, INC.
Condensed Consolidated Statements of Operations
(Amounts in thousands except share and per share data)
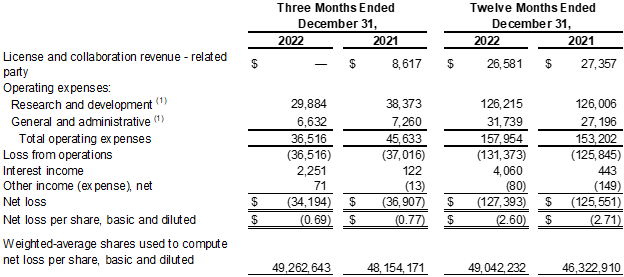
(1) Amount includes non-cash stock-based compensation expense.
PROTAGONIST THERAPEUTICS, INC.
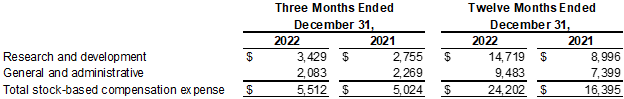
Stock-based Compensation
(In thousands)
PROTAGONIST THERAPEUTICS, INC.
Selected Consolidated Balance Sheet Data
(In thousands)

About Protagonist
Protagonist Therapeutics is a biopharmaceutical company with peptide-based new chemical entities rusfertide and JNJ-2113 (formerly PN-235) in advanced stages of clinical development, both derived from the Company's proprietary technology platform. Rusfertide, a mimetic of the natural hormone hepcidin, is the Company's lead drug candidate currently in a global Phase 3 stage of development. The REVIVE study is now complete, with an open-label extension underway. The global Phase 3 VERIFY study of rusfertide in polycythemia vera is ongoing. Protagonist retains all worldwide development and commercialization rights to rusfertide.
Positive topline results from the FRONTIER 1 study of JNJ-2113 in moderate-to-severe plaque psoriasis became available in March 2023, with further details to be shared at medical meetings starting in the second quarter of 2023. Advancement of JNJ-2113 into a Phase 3 study and meeting the primary endpoint in that study would qualify Protagonist for milestone payments of $50 million and $115 million, respectively. In total, Protagonist remains eligible for up to $855 million in various milestone payments and tiered royalties based on worldwide net drug sales.
More information on Protagonist, its pipeline drug candidates and clinical studies can be found on the Company's website at protagonist-inc.com.
Cautionary Note on Forward-Looking Statements
This press release contains forward-looking statements for purposes of the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Forward-looking statements include statements regarding our intentions or current expectations concerning, among other things, our expectations regarding the potential benefits of JNJ-2113 and rusfertide, timing of JNJ-2113 clinical trials and potential milestones related to JNJ-2113, and our expected cash runway. In some cases, you can identify these statements by forward-looking words such as "anticipate," "believe," "may," "will," "expect," or the negative or plural of these words or similar expressions. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, our ability to develop and commercialize our product candidates, delays or difficulties in enrolling or completing clinical studies, the potential that results from clinical or non-clinical studies indicate our compounds or product candidates are unsafe or ineffective, dependence on third parties to conduct clinical studies and manufacture our products, our ability to earn milestone payments under our collaboration agreement with Janssen Biotech, the impact of the current COVID-19 pandemic on our discovery and development efforts, impact of natural disasters and the impact of the ongoing military conflict in Ukraine and Russia on any future studies, our ability to use and expand our programs to build a pipeline of product candidates, our ability to obtain and maintain regulatory approval of our product candidates, our ability to operate in a competitive industry and compete successfully against competitors that have greater resources than we do, and our ability to obtain and adequately protect intellectual property rights for our product candidates. Additional information concerning these and other risk factors affecting our business can be found in our periodic filings with the Securities and Exchange Commission, including under the heading "Risk Factors" contained in our most recently filed periodic reports on Form 10-K and Form 10-Q filed with the Securities and Exchange Commission. Forward-looking statements are not guarantees of future performance, and our actual results of operations, financial condition and liquidity, and the development of the industry in which we operate, may differ materially from the forward-looking statements contained in this press release. Any forward-looking statements that we make in this press release speak only as of the date of this press release. We assume no obligation to update our forward-looking statements, whether as a result of new information, future events or otherwise, after the date of this press release.
Contacts
Jami Taylor - j.taylor@ptgx-inc.com
SOURCE: Protagonist Therapeutics, Inc.
View source version on accesswire.com:
https://www.accesswire.com/743900/Protagonist-Therapeutics-Reports-Fourth-Quarter-and-Full-Year-2022-Financial-Results-and-Provides-Corporate-Update
