Orally administered LYT-300 achieved a statistically significant (p=0.0001) reduction in the stress hormone response, as measured by salivary cortisol, compared to placebo
This proof-of-concept trial demonstrating a reduction in the physiological stress response supports the further development of LYT-300 as a potential treatment for a range of anxiety disorders
Anxiety disorders affect nearly 30 percent of U.S. adults1, yet current standard-of-care treatments can have drawbacks including mixed efficacy, abuse potential, delayed onset of action and/or poor tolerability; LYT-300 has potential to overcome these challenges as a novel therapeutic in an area of serious patient need
LYT-300 was well-tolerated across the trial with only transient mild or moderate adverse events
PureTech Health plc (Nasdaq: PRTC, LSE: PRTC) ("PureTech" or the "Company"), a clinical-stage biotherapeutics company dedicated to changing the lives of patients with devastating diseases, today announced topline results from its Phase 2a, randomized, placebo-controlled, proof-of-concept trial of LYT-300 (oral allopregnanolone). The trial was designed to evaluate the salivary cortisol response in the Trier Social Stress Test (TSST), a validated clinical model of anxiety in healthy volunteers.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231113556006/en/
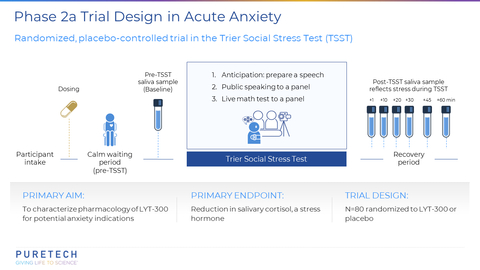
PureTech announced topline results from its Phase 2a, randomized, placebo-controlled, proof-of-concept trial of LYT-300 (oral allopregnanolone). The trial was designed to evaluate the salivary cortisol response in the Trier Social Stress Test (TSST), a validated clinical model of anxiety in healthy volunteers. (Graphic: Business Wire)
Oral administration of LYT-300 achieved the trial’s primary endpoint of a statistically significant reduction versus placebo in the increase from baseline to peak levels of the stress hormone salivary cortisol (p=0.0001). The LYT-300 treatment effect size versus placebo was 0.72, as measured by Cohen’s d, which is one of the most common ways to measure effect size. LYT-300 showed a similar effect size to previously observed results for alprazolam, a benzodiazepine drug indicated for treatment of anxiety disorders, when assessed following the TSST procedure2. An increase in cortisol levels after the TSST is a physiological response and an objective biomarker of acute stress. Eighty healthy volunteers were randomized and treated with either LYT-300 or placebo in a 1:1 ratio. LYT-300 was well tolerated, with all treatment-related adverse events transient, mild or moderate and consistent with the known pharmacology profile of allopregnanolone. Additional data from the study will be presented in a scientific forum.
“Anxiety disorders are an area of significant unmet medical need and current standard-of-care treatments leave much room for improvement due to inconsistent efficacy and adverse events. We know that benzodiazepines, like alprazolam, can reduce the salivary cortisol response to stress in the TSST. Cortisol is an important marker of the physiological response to stress, and reduction of stress overreactivity may be an important mechanism for treating anxiety and stress-related disorders,” said Murray Stein, MD, MPH, FRCPC, Distinguished Professor of Psychiatry and Public Health at the University of California San Diego and an advisor to PureTech. “LYT-300, a non-benzodiazepine neurosteroid, blunts this stress response, highlighting its novel pharmacology and potential for helping patients in serious need of new treatment options.”
“These data validate that LYT-300 has potential to make a difference for people living with anxiety, where there’s been a dearth of innovation and existing treatments have drawbacks,” said Daphne Zohar, Founder and Chief Executive Officer of PureTech Health. “The successful outcome of this trial builds on our strategy of identifying drugs with proven clinical efficacy but with historical limitations that have held back their therapeutic use, and then applying an innovative solution to enhance their potential for patients. In the CNS arena, we previously applied this strategy to invent KarXT for the treatment of schizophrenia. Building on this approach, we now have seven wholly owned CNS programs powered by our Glyph™ platform, which is designed to enable the oral bioavailability of drugs with high first-pass metabolism and resolve hepatotoxicity.”
LYT-300 is an oral prodrug of allopregnanolone, an endogenous neurosteroid. Allopregnanolone has been recognized for its potential to treat a range of neurological and neuropsychiatric indications with a well-established rapid onset of action in mood disorders. The major hurdles associated with endogenous neurosteroids in the past have been their lack of oral bioavailability and inability to chronically administer them to patients, which means they otherwise can only be administered via a long, cumbersome intravenous infusion. To overcome the challenges with this method of administration, medicinal chemistry approaches have been applied to synthesize orally bioavailable chemical analogs of allopregnanolone. These oral analogs may have different pharmacological effects than endogenous allopregnanolone and therefore may not capture its full therapeutic potential. LYT-300 is designed to achieve oral administration of an endogenous allopregnanolone that has the potential to capture the breadth of the natural biological response.
“The data generated with PureTech’s LYT-300 suggest this may be a promising treatment for anxiety disorders, as well as a range of related neurological and neuropsychiatric conditions,” said Maurizio Fava, MD, Psychiatrist-in-Chief at Massachusetts General Hospital and an advisor to PureTech. “The Glyph platform and the therapeutic candidates being developed at PureTech have the potential to treat a range of central nervous system disorders.”
These results further validate PureTech’s Glyph platform, which is designed to employ the lymphatic system's natural lipid absorption and transport process to enable the oral administration of certain therapeutics that otherwise cannot be administered orally. PureTech has generated seven CNS programs based on its Glyph platform, including LYT-300 and LYT-310 (oral cannabidiol), which is currently in development for the treatment of epilepsies and other neurological indications.
As part of an overall development strategy in anxiety-related indications, PureTech will be conducting additional studies in 2024. Further guidance will be provided regarding 2024 catalysts associated with the planned studies.
About LYT-300
LYT-300 is a clinical-stage therapeutic candidate that is in development as a potential treatment for neurological and neuropsychiatric conditions, including anxiety disorders, mood disorders and Fragile X-associated Tremor/Ataxia Syndrome. Developed using PureTech’s Glyph™ technology platform, LYT-300 is an oral prodrug of endogenous allopregnanolone that is designed to overcome its poor oral bioavailability. PureTech completed a randomized, placebo-controlled, Phase 2a, proof-of-concept trial of LYT-300 using a validated clinical model of anxiety in healthy volunteers in 2023, which demonstrated a statistically significant reduction in stress response, as measured by salivary cortisol. PureTech also completed a Phase 1 clinical trial of LYT-300 in 2022, which demonstrated oral bioavailability, tolerability and ?-aminobutyric-acid type A (GABAA) receptor target engagement in healthy volunteers.
About the Glyph™ Platform
Glyph is PureTech's lymphatic-targeting platform which is designed to employ the lymphatic system's natural lipid absorption and transport process to enable the oral administration of certain therapeutics. Glyph reversibly links a drug to a dietary fat molecule, creating a novel prodrug. The linked fat molecule re-routes the drug's normal path to the systemic circulation, bypassing the liver and instead moving from the gut into the lymphatic vessels that normally process dietary fats. PureTech believes this technology has the potential to provide a broadly applicable means of enhancing the bioavailability of certain orally administered drugs that would otherwise be limited by first-pass liver metabolism. PureTech is accelerating development of a Glyph portfolio that leverages validated efficacy, prioritizing highly characterized drugs to evaluate the ability of the Glyph technology to improve oral bioavailability or lymphatic targeting. PureTech's lead Glyph therapeutic candidate, LYT-300 (oral allopregnanolone), completed a randomized placebo-controlled, Phase 2a, proof-of-concept trial using a validated clinical model of anxiety in healthy volunteers in 2023. A Phase 2 clinical trial of LYT-300 in FXTAS in collaboration with the University of California, Davis, is expected to initiate in 2024. PureTech has now generated seven CNS programs based on the Glyph platform. PureTech has a robust intellectual property (IP) portfolio that includes licensed patents as well as wholly owned IP, covering the Glyph technology platform, which is based on the pioneering research of Christopher Porter, PhD, and his research group at the Monash Institute of Pharmaceutical Sciences at Monash University. The Porter Research Group and collaborators have published research in Nature Metabolism, Angewandte Chemie and the Journal of Controlled Release supporting the Glyph platform's ability to directly target the lymphatic system with a variety of therapies.
About the Trier Social Stress Test
The Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993) is a validated clinical model of anxiety. It is a widely used and well-established psychological and physiological laboratory test designed to induce acute psychosocial stress in human participants. It is considered the gold standard in human experimental stress research and is used by researchers to investigate how individuals respond to acute stressors, how stress affects cognitive and emotional processes, and how stress might contribute to various psychological and physiological conditions. The TSST puts the participant in situations designed to elicit unpredictable, novel, anticipatory, and social stress such as preparing and giving a speech, performing arithmetic, and being observed by judges. It models stress reactivity, which is an important component in many mood, stress and anxiety disorders, and the TSST robustly increases physiological markers of stress including salivary cortisol. Benzodiazepines, a clinically effective drug class indicated for the treatment of anxiety, reliably blunt the increased salivary cortisol in the TSST.
Juliane Hellhammer, PhD, founder and CEO of daacro (a contract research organization specialized on psychotropic drug effects) and recognized expert on the Trier Social Stress Test, is an advisor to PureTech.
About PureTech Health
PureTech is a clinical-stage biotherapeutics company dedicated to giving life to new classes of medicine to change the lives of patients with devastating diseases. The Company has created a broad and deep pipeline through its experienced research and development team and its extensive network of scientists, clinicians and industry leaders that is being advanced both internally and through its Founded Entities. PureTech's R&D engine has resulted in the development of 27 therapeutics and therapeutic candidates, including two (Plenity® and EndeavorRx®) that have received both US FDA clearance and European marketing authorization and a third (KarXT) that has been filed for FDA approval. A number of these programs are being advanced by PureTech or its Founded Entities in various indications and stages of clinical development, including registration enabling studies. All of the underlying programs and platforms that resulted in this pipeline of therapeutic candidates were initially identified or discovered and then advanced by the PureTech team through key validation points.
For more information, visit www.puretechhealth.com or connect with us on X (formerly Twitter) @puretechh.
Cautionary Note Regarding Forward-Looking Statements
This press release contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements contained in this press release that do not relate to matters of historical fact should be considered forward-looking statements, including without limitation those statements that relate to our expectations around the design of and the timelines and key milestones associated with clinical trials for LYT-300 and other programs from the Glyph™ platform, including in anxiety-related indications, the therapeutic potential of LYT-300, our expectations regarding the Glyph platform including the potential for new treatment applications, our therapeutic candidates and approach towards addressing major diseases, and our future prospects, developments, and strategies. The forward-looking statements are based on current expectations and are subject to known and unknown risks, uncertainties and other important factors that could cause actual results, performance and achievements to differ materially from current expectations, including, but not limited to, those risks, uncertainties and other important factors described under the caption "Risk Factors" in our Annual Report on Form 20-F for the year ended December 31, 2022 filed with the SEC and in our other regulatory filings. These forward-looking statements are based on assumptions regarding the present and future business strategies of the Company and the environment in which it will operate in the future. Each forward-looking statement speaks only as at the date of this press release. Except as required by law and regulatory requirements, we disclaim any obligation to update or revise these forward-looking statements, whether as a result of new information, future events or otherwise.
1] Any Anxiety Disorder. (n.d.). National Institute of Mental Health (NIMH). https://www.nimh.nih.gov/health/statistics/any-anxiety-disorder
2] Psychoneuroendocrinology, 31(10), 1278–1288. https://doi.org/10.1016/j.psyneuen.2006.09.009 .

View source version on businesswire.com: https://www.businesswire.com/news/home/20231113556006/en/