Focusing on novel molecular hydrogen therapies for brain health
TORONTO, Oct. 30, 2024 (GLOBE NEWSWIRE) -- DiagnaMed Holdings Corp. (“DiagnaMed” or the “Company”) (CSE: DMED) (OTCQB: DGNMF), a life sciences company focused on brain health, announces its strategic initiative in advancing therapies that unlock the medical potential of molecular hydrogen for brain health.
Hydrogen is well-known for its industrial use as a pollution-free fuel. The global hydrogen generation market size was estimated at USD 170.14 billion in 2023 and is expected to grow at a compound annual growth rate (CAGR) of 9.3% from 2024 to 20301.
Molecular hydrogen therapy is a growing field and poised for rapid clinical adoption. There are over 2,000 scientific publications on molecular hydrogen’s potential therapeutic effects in over 100 human studies2. Molecular hydrogen has been clinically demonstrated to provide antioxidant, anti-inflammatory and neuroprotective effects. It can potentially aid in managing chronic diseases by diminishing oxidative stress and the associated inflammatory pathways. The cellular bioavailability of molecular hydrogen is high3 and has the potential for antiaging, cardiovascular disease, metabolic syndrome (i.e. diabetes, obesity), and neurodegenerative disorders (i.e. Parkinson’s and Alzheimer’s disease)4.
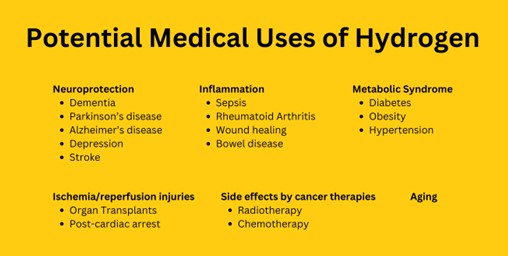
Figure #1: Molecular hydrogen as a preventive and therapeutic medical gas
Although molecular hydrogen has a high safety profile, proven antioxidant and anti-inflammatory effects, known mechanism of action and viable delivery options (i.e. inhalation, infusion, ocular, topical, and oral), its clinical use is limited primarily due to its low hydrogen content and rapid evaporating action in water, resulting in a lower effective concentration and stability6. In addition, hydrogen inhalation and hydrogen-rich water machines are expensive, bulky and complex to use, making it challenging to determine the appropriate dosage for desired clinical outcomes.
According to a published article titled “Molecular hydrogen therapy for neurological diseases: a review of current evidence,” a number of studies have demonstrated the neuroprotective effects of hydrogen therapy in stroke, neurodegenerative diseases, neurotrauma, and global brain injury4. Also, no adverse effects have been reported in the human studies related to the administration of hydrogen therapy and its clinical use as an adjunctive treatment of various neurological diseases is promising5.
Aligned with the Company’s objective of providing therapeutic and diagnostic solutions for brain health, DiagnaMed plans to initially develop and commercialize novel molecular hydrogen therapies specifically tailored for neurological disorders. These therapies will offer different doses, durations, and methods of administration. DiagnaMed intends to partner with companies that have or are exploring ‘white’ hydrogen sources and licensing technologies from academic institutions that enable natural and simulated hydrogen to meet the diverse needs of patients.
“Molecular hydrogen therapy has promising potential for clinical use in various diseases and may fill the gap in providing natural, safe and potentially efficacious solutions for brain health,” said Fabio Chianelli, CEO of DiagnaMed. “Proposed molecular hydrogen-based products aim to complement our BRAIN AGE® Brain Health AI Platform for improving brain health. I look forward to updating the public on our product developments in unlocking the medical potential of hydrogen and partnering with companies and academic institutions harnessing the promise of natural hydrogen.”
About DiagnaMed
DiagnaMed Holdings Corp. (CSE: DMED) (OTCQB: DGNMF) is a life sciences company focused on developing and commercializing novel therapies and diagnostics using AI for brain health. DiagnaMed is commercializing BRAIN AGE® Brain Health AI Platform, a world-first consumer brain health and wellness AI solution that estimates brain age and provides a brain health score. In addition, the Company is exploring the medical use of hydrogen for brain health. Visit DiagnaMed.com.
For more information, please contact:
Fabio Chianelli
Chairman and CEO
DiagnaMed Holdings Corp.
Tel: 416-800-2684
Email: info@diagnamed.com
Website: www.diagnamed.com
Neither the Canadian Securities Exchange nor its Regulation Services Provider have reviewed or accept responsibility for the adequacy or accuracy of this release.
Cautionary Statement
Certain statements in this news release are forward-looking statements, including with respect to future plans, and other matters. Forward-looking statements consist of statements that are not purely historical, including any statements regarding beliefs, plans, expectations or intentions regarding the future. Such information can generally be identified by the use of forwarding-looking wording such as “will”, “may”, “expect”, “could”, “can”, “estimate”, “anticipate”, “intend”, “believe”, “projected”, “aims”, and “continue” or the negative thereof or similar variations. The reader is cautioned that assumptions used in the preparation of any forward-looking information may prove to be incorrect. Events or circumstances may cause actual results to differ materially from those predicted, as a result of numerous known and unknown risks, uncertainties, and other factors, many of which are beyond the control of the Company, including but not limited to, business, economic and capital market conditions, the ability to manage operating expenses, and dependence on key personnel. Such statements and information are based on numerous assumptions regarding present and future business strategies and the environment in which the Company will operate in the future, anticipated costs, and the ability to achieve goals. Factors that could cause the actual results to differ materially from those in forward-looking statements include, the continued availability of capital and financing, litigation, failure of counterparties to perform their contractual obligations, loss of key employees and consultants, and general economic, market or business conditions. Factors that could cause actual results to differ materially from those anticipated in these forward-looking statements are described under the caption “Risk Factors” in Company’s management’s discussion and analysis for the three and nine months ended June 30, 2024 (“MD&A”), dated August 22, 2024, which is available on the Company's profile at www.sedarplus.ca. Forward-looking statements contained in this news release are expressly qualified by this cautionary statement. The reader is cautioned not to place undue reliance on any forward-looking information. The forward-looking statements contained in this news release are made as of the date of this news release. Except as required by law, the Company disclaims any intention and assumes no obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise.
This news release does not constitute an offer to sell or the solicitation of an offer to buy, and shall not constitute an offer, solicitation or sale in any state, province, territory or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state, province, territory or jurisdiction.
Footnotes:
- Grandviewresearch.com. Hydrogen Generation Market Size, Share & Trends Analysis Report By System (Merchant, Captive), By Technology (Steam Methane Reforming, Coal Gasification), By Application, By Source, By Region, And Segment Forecasts, 2024 - 2030. [(accessed on 30 October 2024)]. Available online: https://www.grandviewresearch.com/industry-analysis/hydrogen-generation-market.
- Ichihara, M., et al. Beneficial biological effects and the underlying mechanisms of molecular hydrogen-Comprehensive review of 321 original articles. Med. Gas Res. 2015, 5, 12.
- Nicolson, GI., et al. 2016. Clinical Effects of Hydrogen Administration: From Animal and Human Diseases to Exercise Medicine. Int. J. Clin. Med. 7(1): 32-76. Doi:10.4236/ijcm.2016.71005.
- Shigeo Ohta, Molecular hydrogen as a preventive and therapeutic medical gas: initiation, development and potential of hydrogen medicine, Pharmacology & Therapeutics,Volume 144, Issue 1, 2014, Pages 1-11, ISSN 0163-7258, https://doi.org/10.1016/j.pharmthera.2014.04.006.
- Ramanathan D, Huang L, Wilson T, Boling W. Molecular hydrogen therapy for neurological diseases: a review of current evidence. Med Gas Res. 2023 Jul-Sep;13(3):94-98. Doi: 10.4103/2045-9912.359677. PMID: 36571372; PMCID: PMC9979207.
- LeBaron, et al. Electrolyzed Reduced Water: Review II: Safety Concerns and Effectiveness as a Source of Hydrogen Water. Int. J. Mol. Sci. 2022, 23, 14508.
A photo accompanying this announcement is available at https://www.globenewswire.com/NewsRoom/AttachmentNg/f1fd1465-a3ba-4117-97ee-c29f94c80003


