
We are initiating coverage on INmune Bio Inc. (INMB:NASDAQ). According to the company, INmune is "Reprogramming the Innate Immune System for the Treatment of Diseases." On the surface, this sounds like a fairly standard biotech tagline, but there's a more here than meets the eye; by dealing with the innate immune system, INmune has the potential to treat multiple diseases with the same therapy. As such, this small company is targeting several large opportunities simultaneously.
INmune targets diseases that have end markets in the tens to hundreds of billions of dollars. To date their clinical data has been solid in all trials. Meanwhile, insiders have funded, and continue to fund, a significant portion of the company's cash needs. In this report, we will delve into the company, its target markets, its approach to these markets and its products.
The Innate Immune System
It's a little tough to get your head around the thought that this little $42 million market cap company, INmune, is going after three markets simultaneously, and that those three markets are some of the largest potential markets out there.
"You've never seen a company that has cancer, Alzheimer's and NASH, ever before probably. Why can we do that? Because we're dealing with inflammation and chronic inflammation plays a critical role in those diseases." ~ David Moss, CFO
By going after cancer, Alzheimer's disease (AD) and NASH (Nonalcoholic steatohepatitis), the company is targeting three totally different diseases that have a very common trait in their biologics. However, these are also areas of biotech that are crowded with well-funded competition and many therapies in development. What is INmune doing differently and how can it compete?
To answer this question let's take a few steps back and look at human biology and the beginning of biotech as an industry. Starting with human biology, there is within each of us an immune system. The immune system is highly complex but can really be broken down into two constituents: the innate system and the adaptive system.
The innate immune system is a front-line defense against disease that gets its name from the fact that we are all born with it already in place, and it changes little throughout our lives. It provides protection by recognizing general features of possible pathogens. Pathogens would be any foreign invader that can cause disease. An example of the innate system is your skin, which blocks entry of many kinds of organisms. Cells of the innate immune system recognize general features of pathogens.
These cells of the innate system do not distinguish within the various classes of pathogens, instead attacking all of them. To use a military analogy, it would be like using the same type of missile to shoot at many different kinds of targets instead of having different missiles for different types of target. Macrophages, for instance, are cells that participate in the innate immune response by finding, eating and killing many different types of bacteria. Natural killer cells (NK cells) are another type of innate immune cell that function to eliminate cells that have become infected with viruses and cancer cells.
The adaptive immune system is more of a rifle shot approach to defending the body against individual pathogens. Unlike the innate immune system, the acquired immune system is highly specific to each particular pathogen. The adaptive system creates immunological memory after an initial response to a specific pathogen and leads to an enhanced response to subsequent encounters with that pathogen.
In a nutshell, the human immune system has its innate side that is a front line against everything bad for the body and its adaptive side that adapts to and targets specific pathogens. These targeted pathogens are diseases that have somehow made it past the innate system. This could be due to a heavy dosage, like a smoker developing lung cancer due to years of overwhelming his system, or it could be due to dysfunction of the innate system.
Since the beginning of the biotechnology industry, scientists have known about both sides of the immune system. This is still a young industry, however, and researchers have been strictly focused on the adaptive side of the immune system due to early success there. When you look at the biggest and most successful biotech companies to date, they are all focused on the adaptive immune system. Checkpoint inhibitors or T-cell therapies, for example, are therapies targeting adaptive immune defenses.
Thus, by definition, these firms and their research dollars have been a rifle shot after individual pathogens or diseases. As an example, CAR-T therapy has been very successful in targeting specific types of cancer by working on T-Cells, a part of the adaptive system. This has worked well as companies focus on taking care of one disease at a time with their drug programs.
It's interesting to note that not all mammals have both sides of the immune system found in humans. Most only have an innate system, not the adaptive side. Yet, many animals with only an innate system don't get diseases such as cancer. Researchers who have been focused on the adaptive system have really ignored an equally important defense in the innate system.
INmune is pursuing a radically different approach from the majority of other biotechs. As we mentioned earlier, the innate side of the immune system is like a missile that can work on any kind of target. INMB is focused on "reprogramming the innate immune system." By working on the side of the immune system that can deal with any kind of pathogen, INmune has the potential to be working on products that span many different classes of disease. It is through this approach that INmune can afford to have a cancer, Alzheimer's and NASH study all going on at the same time. It's a different approach than most are taking and, as a result, is has the potential for broad-reaching success.
The Products
INmune has four programs in its business plan. These target three different therapeutic areas and come from two platforms the company is developing. Here's the product roadmap from their recent presentation at the HC Wainwright Conference (btw, the webcast is a great resource).
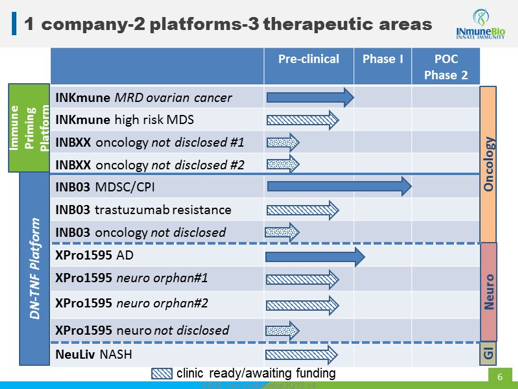
It's a large bite to swallow, targeting GI, neuro and oncology at the same time. However, here's what Dr. R.J. Tesi, the company's CEO, calls its "dirty little secret"; INB03, XPro1595 and NeuLiv, which is its "DN-TNF Platform," are all the same product, just being tested in different indications. When the innate immune system becomes dysfunctional, reprogramming it can lead to benefits in many of the areas where it's effective. Which means that a therapy targeting the innate immune system can be effective across a broad swath of pathogens and, thus, many target indications.
Looking at these diseases, the market opportunity being addressed by both platforms is massive.

Success in any one of these areas would obviously lead to a great outcome for investors. But, what gives INmune any confidence that success is going to be achieved, particularly across these three indications with one DN-TNF therapy targeting them? It's the method of action that the drug brings to the patients. Its drug is an off-the-shelf version of a novel TNF inhibitor, a depiction of which is below.
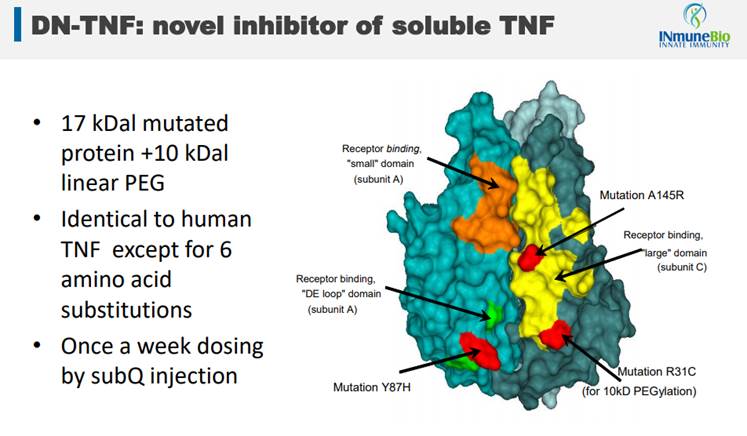
It's the three red dots on the image above that differentiates INmune's drug from soluble TNF found in humans and from TNF inhibitors (blockbuster drugs like Humira, Enbrel and Remicade) that have been around for a while and are very popular drugs. TNF inhibitors are drugs that help stop inflammation. They're used to treat diseases like rheumatoid arthritis (RA), juvenile arthritis, psoriatic arthritis, plaque psoriasis, ankylosing spondylitis, ulcerative colitis (UC) and Crohn's disease.
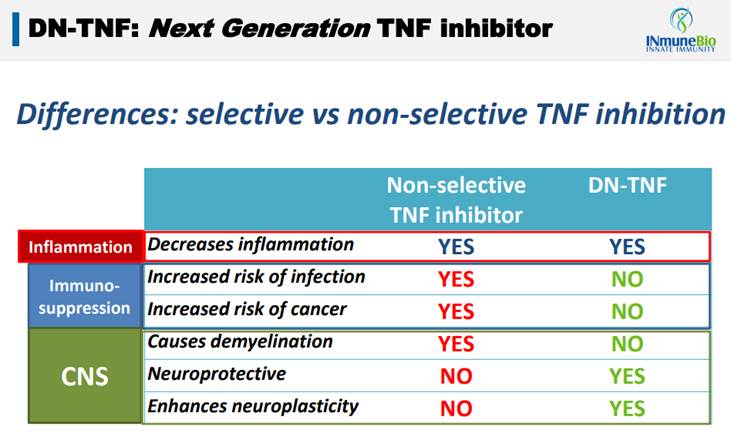
Inflammation plays a role in cancer and other diseases. However, because of the side effects of the existing TNF inhibitors suppressing a patients' immune system, they can't be used. INmune believes that with its targeted DN-TNF inhibitor, which is designed to remove the side effects of the existing TNF drugs, they can greatly expand the market opportunity while reducing the number of patients suffering side effects from the treatment. Thus, they can go after markets like cancer and neuro where the existing TNF players can't.
And, that's just in cancer patients. Alzheimer's has been directly linked to inflammation as is NASH. A targeted therapy that blocks the "bad" TNF has possibilities across many diseases. The innate immune system hasn't been working in these patients and this therapy has the potential to re-energize it.
The problem with other TNF inhibitors, and this is a very large class of drugs, is that they are non-selective. There are two kinds of TNF found in humans, Soluble TNF and Trans-Membrane TNF, and the existing class of drugs attacks both of these. This is why they are good at stopping inflammation (blocking Soluble TNF stops inflammation) but carry with them a plethora of side effects. Because, by blocking Trans-Membrane TNF, patients have increased risk of diseases like cancer, infections and CNS disease.
INmune's drug is not a front-line therapy per se. But it does do a better job of treating inflammation caused by Soluble TNF while allowing the important Trans-Membrane TNF to do its job. In this way INmune's first platform has the potential to be an important adjunct therapy in many diseases that have inflammation as an associated problem with curing them, which is how it has multiple treatments undergoing clinical testing.
INKmune, the other therapeutic platform under development, works with NK cells in the innate immune system. Before the adaptive systems is even aware of a threat to our body, the Innate immunity is acting as our first line of defense against infection and cancer. Around 10% of the lymphocytes in our peripheral bloodstream are part of the innate response, and 95% of those are natural killer (NK) cells. Each of these innate killer cells can recognize, target and kill harmful cells through recognition of proteins and glycoproteins, which are expressed on "stressed" cells, such as tumor and virally infected cells.
Why is innate immunotherapy likely to improve outcomes? Cancer cells are constantly arising in our bodies as a consequence of random genetic mutations, some of which give a cell a survival or proliferation advantage. These random changes are mostly novel and have never been presented to the adaptive immune system. The only cells able to detect the "stress" signals of a tumor cell that is dividing too frequently are NK cells, which can eliminate tumor cells before they become a clinically detectable cancer.
However, eventually cancer cells get smart and they evade NK cell killing. By reprogramming signals on the cancer cells surface they are able to avoid NK cell detection. INKmune provides these missing signals to the patient's own natural killer cells to help them target tumors, unlike how CAR-T works on T-Cells…but without having to individualize treatment and that associated expense.
As this slide from their presentation shows, INKmune primed cells recognize cancer and overwhelm it. By altering the NK cells, INKmune (TpNK) turns them into cancer killers.
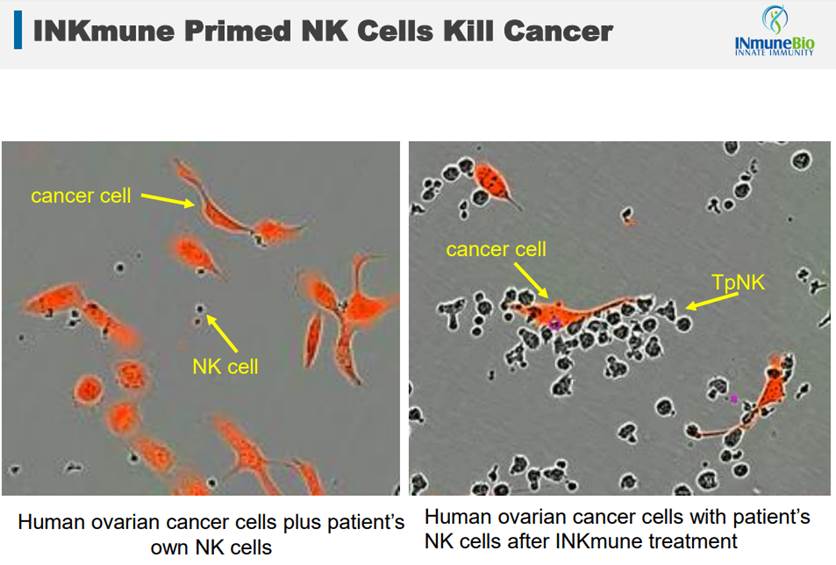
Similar to the other platform, INKmune is likely to be a combination therapy. The best use case will be found in cancer victims who have a high likelihood of recurrence. This is a frequent case in situations like breast cancer, where many survivors get another form of cancer after several years. In these situations, taking INKmune as an ongoing therapy could be a possible preventative measure against a return of cancer.
Success to Date and Plans for Future Trials
So far we've discussed the immune system, what the innate immune system is and why INmune is pursuing a strategy of focusing here, and the two platforms that the company is developing. Now let's turn our attention to the progress that has been made by INmune as this is where the story starts to get very exciting.
There are very few, if any, biotech companies in phase 1 clinical trials that have over 70 published reports on their technology. The large number of research institutions that have done work with either of these platforms is testament to the possibilities of the underlying science. More importantly, the fact that early stage testing has been consistent and positive across both product lines is highly encouraging.
Focusing on the DN-TNF product line, containing XPro1595, INB03 and Neuliv, there have been over 60 research papers published. A complete list of them is available on INmune's website.
INB03, INmune's cancer drug, has completed a phase 1 trial, the interim results of which were released in August. Final results from this trial are expected to be released in the first half of December. Here's what the company had to say about the interim results…
"No drug-related serious adverse events have been reported, and INB03 was well tolerated…The inflammatory cytokine IL6, a biomarker of soluble TNF function, decreased by more than 50% in half of the patients, suggesting a pharmacodynamic effect of INB03."
Based on these statements it stands to reason that the final results will be positive. In addition, the company is already working on its phase 2 trial design, which should also be released soon, further suggesting positive results are coming.
Furthermore, based on prior history of other TNF inhibitors, it stands to reason that the drug will be successful. This product is also a TNF inhibitor, albeit different from the others in the market. TNF inhibitors are one of the leading classes of drugs (on sales dollars) sold in the world and their effectiveness is well understood. The fact that, in its phase 1 safety trial, INmune saw suggestions of a pharmacodynamic effect says that the product is simply working as one would expect it to.
Meanwhile XPro1595 has also demonstrated effectiveness in animal studies and is in the middle of a phase 1a trial at this time, with a phase 1b having started this week with the first patient being injected. With recent data showing correlation between Alzheimer's disease and inflammation, it stands to reason that XPro1595 could be effective here. Remember, XPro1595 and INB03 are the same drug, just going through trials for different diseases. If those diseases share a root problem of inflammation and one drug works on inflammation, the other should as well; the positive pharmacodynamic effect of INB03 bodes extremely well for its partner drugs.
Finally, the other platform drug, INKmune, has also received a fair amount of research attention, with similar positive results. INmune expects to launch the phase 1 trial of INKmune shortly.
Insider Support and Strong Balance Sheet
Having had nothing but consistently positive results, INmune appears to be a path toward clinical success and, hopefully, eventual success in treating sick patients with FDA approved products. But, it's early stage and there's more to a company than simply clinical success, a lesson I've learned the hard way in some recent investments.
Beyond the clinic, the quality of the management and having deep pocketed investors willing to back a company through their cash-burning early stages is equally important to the success of a company. Without access to funds from investors who are fundamental in nature and willing to fund a company in a clean transaction at a decent valuation, a stock can suffer tremendously. This can happen even in the face of strong data.
INmune is a company that has done all the right things in its public market strategy, in my opinion. The balance sheet is pristine. The company has been funded through straight equity with zero debt or converts in place.
More exciting is the fact that insiders have put a lot of capital into this company. After INmune went public at $8 per share the insiders, including management and the board, put an additional $5 million into the company's coffers. At $9 per share! And, they have been adding to their holdings in the open market more recently, as the stock has trended lower.
By the way, potential investors might want to check out the bios of management and the board. This is a seasoned team with a track record of success. They also have the financial wherewithal to continue funding the company if necessary.
I stated earlier that the interim results bode well for success in the ongoing trials. However, remember that these early trials are not double-blind; the data is open to the company all along. Thus, insider buying, like we've been seeing, could be an even bigger indicator as to how things are progressing.
The Bottom Line
Investing in biotech is not without risks. These risks, generally speaking, relate to the clinical success of a company and its ability to fund the business. As companies progress to later stage, both of these risks diminish to some degree and the share price rallies. Before this happens, however, early-stage biotech stocks can languish. This creates opportunity for those investors who are willing to take the risk of investing in an early stage company.
INmune is, potentially, one of those rare opportunities where the market is pricing in risk that may not exist to the degree the market seems to think exists. The clinical data to date for INmune has been very strong. These trial results have been consistent across more than 60 published papers. Yes, there is clinical risk here, but it certainly seems that the plethora of data out there suggests the products stand a better than average chance of success.
Meanwhile, the company will indeed require more funding, but this may not be as substantial an overhang as is typical in micro-cap biotechs. The funding required is not in the hundreds of millions, rather in the $15 million or so amount. Insiders have deep pockets and don't look forward to dilution at levels well below where they just invested $5 million. It's very conceivable that funding risk will be removed by insider participation once again.
I see INMB as a potential platform company that can, through its dealing with the innate immune system, develop products that work alongside existing therapies across many diseases. The areas of treatment are huge, the products have great results to date and insiders are strong and committed to this company. INmune has all the hallmarks of possibly being that one in a million micro-cap biotech and Tailwinds is excited to be covering the stock and following its progress.
Daniel Carlson is the founder and managing member of Tailwinds Research Group and its parent company DFC Advisory Services, which is a licensed registered investment advisor (CRD # 297209). Tailwinds is a microcap focused research company that provides research on and consults to over 20 emerging growth companies in the technology and life sciences arenas. DFC Advisory Services is an RIA that manages money dedicated to investing in the companies covered by Tailwinds. For more information on these two companies and their track record, please see www.tailwindsresearch.com. Prior to founding these two entities, Dan spent many years working with small public companies, having been CFO of two public companies and helping finance many others. A 1989 graduate from Tufts University with a degree in Economics, Dan’s formative years in business were spent as an equity trader, first on the Pacific Coast Stock Exchange then on the buyside at several multi-billion dollar firms.
This article was submitted by Tailwinds Research. For more information on Tailwinds Research or on INmune Bio, please visit www.tailwindsresearch.com.
Tailwinds owns stock in INmune Bio. For a complete list of disclaimers and disclosures, please click here.
Disclosure:
1) Daniel Carlson: I, or members of my immediate household or family, own shares of the following companies mentioned in this article: INmune Bio. I personally am, or members of my immediate household or family are, paid by the following companies mentioned in this article: None. My company has a financial relationship with the following companies referred to in this article: INmune Bio. Additional disclosures and disclaimers are above. I determined which companies would be included in this article based on my research and understanding of the sector.
2) The following companies mentioned in this article are billboard sponsors of Streetwise Reports: None. Click here for important disclosures about sponsor fees. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
3) Statements and opinions expressed are the opinions of the author and not of Streetwise Reports or its officers. The author is wholly responsible for the validity of the statements. The author was not paid by Streetwise Reports for this article. Streetwise Reports was not paid by the author to publish or syndicate this article. Streetwise Reports requires contributing authors to disclose any shareholdings in, or economic relationships with, companies that they write about. Streetwise Reports relies upon the authors to accurately provide this information and Streetwise Reports has no means of verifying its accuracy.
4) This article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their immediate families are prohibited from making purchases and/or sales of those securities in the open market or otherwise from the time of the interview or the decision to write an article until three business days after the publication of the interview or article. The foregoing prohibition does not apply to articles that in substance only restate previously published company releases.