
Even more so than cancer, it’s the one disease that we all fear the most. The thought of falling prey to Alzheimer’s disease and to the inevitable desecration of our mind is something that makes even the bravest among us shudder.
After all, if we’re robbed of our sense of who we really are, we are doomed to live our last days without the dignity that defines us and that we hold most dear. The ultimate horror of Alzheimer’s is that it is as indiscriminate, merciless, and as devastating as a wind-swept California wildfire.
It is therefore not surprising that a disease-modifying treatment for Alzheimer’s has become a Holy Grail of sorts in the biotech industry. It’s now a disease so ubiquitous that it casts a shadow over everyone’s family. No one is spared. So too does it exact a devastating financial toll on society – perhaps even greater than cancer – with Alzheimer’s patients needing around-the-clock care for an average of eight years, while some suffer for up to 20 years.
The estimated cost for caring for Americans with Alzheimer's and other dementias is well in excess of a quarter of a trillion dollars per annum. This figure doesn’t even include unpaid caregiving. Also, Alzheimer's is ranked as the third leading cause of death of seniors in the United States, surpassed only by heart disease and cancer. Around 6 million Americans have become victims of this disease, and this number rises each year as lifespans increase due to advancements in medical science.
Such unnerving considerations help explain why the capital markets are willing to rally behind companies with in-development Alzheimer’s drug therapies, especially as a reaction to positive clinical data from in human trials. Lately, several such companies have seen their share prices surge higher.
Alzheimer’s Disease Drug Candidates Light Up Capital Markets
Consider high-flying pharmaceutical company Biogen (NASDAQ: BIIB), which is based in Cambridge, Massachusetts. It recently experienced a much-publicized reversal of fortunes with the announcement that trials for an advanced-stage Alzheimer’s drug candidate, known as aducanumab, were back on track.
Having languished for months after the company suffered a short-lived setback with its trials earlier this year, Biogen’s share price powered higher on news that its drug candidate is one step closer to commercialization with word on the street that Biogen may be filing for FDA approval shortly.
This is arguably the biggest breakthrough after 40 years of efforts to find an effective drug to treat Alzheimer’s. If successful, Biogen could initially dominate a $10 billion market for treating Alzheimer’s, according to analysts. This is notwithstanding the fact that Biogen’s drug candidate is only meant to decelerate the spread of early-stage Alzheimer’s, rather than stopping it dead in its tracks. It does so by clearing away the clumps of the plaque that cloud the brains of Alzheimer’s sufferers.
Another company is working on what may actually be a more important breakthrough for Alzheimer’s and other afflictions that are defined by serious nerve damage – extraordinary though as this may sound. Vancouver-headquartered
NervGen Pharma Corp. (TSX-V: NGEN) (OTCQX: NGENF) appears to be punching well above its weight in this endeavour. Though it has its corporate office in Canada, the company’s IP was developed by one of the world’s leading medical researchers of nerve damage from spinal cord injury.
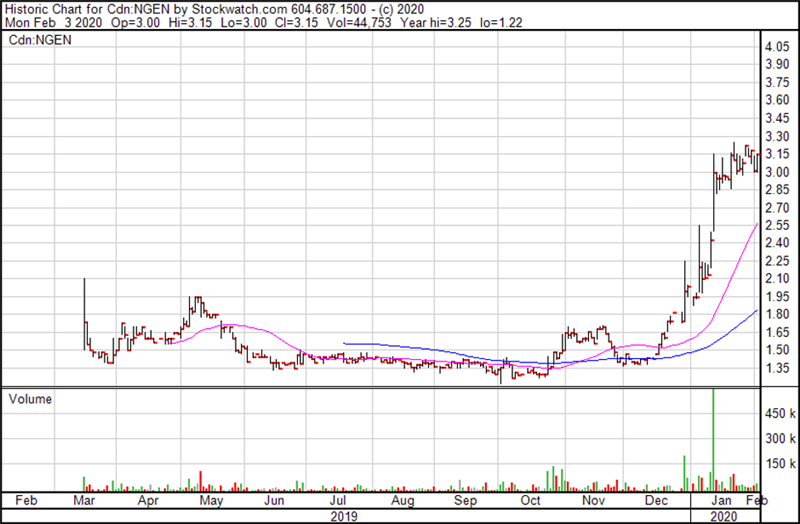 Nervgen’s share price has been a stellar performer lately due to the promise of its drug candidate for Alzheimer’s disease.
Nervgen’s share price has been a stellar performer lately due to the promise of its drug candidate for Alzheimer’s disease.
Yet, with a market capitalization of only about US $70 million, NervGen has yet to get on the radar of the mainstream investment community. However, its rising fortunes have not gone completely unnoticed by some savvy biotech investors. To this point, the company’s share price has virtually doubled in recent weeks. And the stock’s trajectory is likely to continue to power forward in 2020 as NervGen moves ever closer to the commencement of human trials later this year. That’s when the blockbuster drug potential of its unique nerve regeneration therapy will likely start to garner headlines.
Could This Be Modern Medicine’s Holy Grail?
Until recently, NervGen’s focus has mostly been on developing nerve regeneration for the treatment of spinal cord injuries.
In fact, some remarkable results have been achieved in preclinical trials, including one where the majority of the rodents had a substantial recovery of functionality in their legs after sustaining severe spinal cord damage.
While NervGen has not yet had a chance to try to replicate such profoundly positive results in humans, the medical science world is paying very close attention because there are no known therapies approved that stimulate nerve regeneration. The attention is also driven by the remarkable nature of NervGen’s drug which has numerous mechanisms of action. Not only does it regenerate damaged nerves, but it also creates entirely new nerve connections which is known as plasticity.
In addition, NervGen intends to commence a Phase 2 clinical trial in early 2021 for the treatment of multiple sclerosis. In this regard, the company’s lead drug candidate, known as NVG-291, is a potential treatment for the disease’s debilitating conditions which include: numbness/loss of sensation, chronic and debilitating pain, partial loss of movement, paralysis and even incontinence. This is due to a third mechanism of action of NVG-291 called “remyelination”. This involves reversing cellular damage in the scar tissue that envelopes and traps damaged nerves, allowing them to subsequently repair themselves.
That said, NervGen’s US research team believes that the same nerve-rejuvenating biotechnology can also be adapted to treat Alzheimer’s, rather than just mitigating its symptoms. This is because NervGen’s medical breakthrough is restorative due to the multiple mechanisms of action, rather than merely addressing symptoms. And when big pharma seeks technologies to license, they look for truly novel and innovative approaches of a drug such as NervGen’s which have the potential to be disruptive and has received positive affirmation from several Alzheimer's disease key opinion leaders.
Of particular note, the company’s decision to target its drug therapy candidate in the war against Alzheimer’s disease has been especially well received by investors. As has been the case for Biogen and AC Immune, NervGen’s share price has been trending upward since its announcement.
The essence of the technology is that it unlocks a damaged nervous system's natural ability to repair itself. The proprietary molecules ‘unstick’ nerves and prevent new ones from getting stuck. This is achieved by interfering with synaptic-like connections so the nerves can regrow in places that are normally highly inhibited by scar tissue.
The co-inventor of NervGen's technology is Dr. Jerry Silver, Professor of Neurosciences at Case Western Reserve University's School of Medicine in Cleveland, Ohio. He has been working on this unique approach to nerve rejuvenation biotechnology since the early 90s by focusing on a protein called CSPG that inhibits the body's natural ability to regrow and regenerate.
One of the world’s most foremost neuroscience researchers of spinal cord injury, Dr. Silver is the recipient of the Ameritec Prize for significant accomplishments toward a cure for paralysis, as well as the Christopher Reeve-Joan Irvine Research Medal (The Reeve-Irvine Medal) for his research into damaged spinal cord injuries.
Dr. Silver’s work with NVG-291 is beginning to get the attention of all the right people, now that it might finally be on the cusp of clinical validation. Among them is George Perry, PhD, editor-in-chief for the Journal of Alzheimer's Disease and Professor of Biology and Semmes Distinguished University Chair in Neurobiology at the University of Texas at San Antonio, who calls it “promising.”
Perry adds, “NervGen's platform technology introduces a truly novel approach to treating Alzheimer's disease.”
 Brain damage caused by Alzheimer’s disease demonstrated on the left, compared to a healthy brain on the right.
Brain damage caused by Alzheimer’s disease demonstrated on the left, compared to a healthy brain on the right.
Benefitting From Pharma Industry Backing
The fact that NervGen is addressing a significant unmet medical need for the treatment of nerve damage due to trauma and diseases means that a very lucrative multi-billion dollar marketplace beckons.
This is predicated on the assumption that this drug therapy continues to hold up during clinical trials, which will be ongoing for two to three years, starting in early 2020.
This no doubt explains why NervGen just received a US $1.5 million shot in the arm towards its R&D budget, courtesy of CSBio. The Silicon Valley-based drug manufacturing company advanced the funds as part of a US $3 million manufacturing contract to produce sufficient quantities of NervGen’s drug candidate to facilitate these upcoming clinical trials.
It is well worth reiterating that no drugs have been approved anywhere in the world for nerve regeneration and remyelination, as well as improved plasticity in damaged nerves. Additionally, existing treatments are not considered very effective. So, the stakes are especially high for NervGen in its bid to one day commercialize a blockbuster drug candidate that promises to even outshine Biogen’s advanced-stage, in-development Alzheimer’s drug.
This tantalizing reality is not lost on Radvak who enthuses, “This is a once in a lifetime opportunity to pioneer nerve repairing drug therapies that target some of the most devastating and pervasive diseases known to humankind. It’s both exhilarating and humbling at the same time to realize that we really do seem to have a tiger by the tail.”
ABOUT THE AUTHOR: Marc Davis has a deep background in the capital markets spanning 30 years, having mostly worked as an analyst and stock market commentator. He is also a longstanding financial journalist. Over the years, his articles have also appeared in dozens of digital publications worldwide. They include USA Today, CBS Money Watch, The Times (UK), Investors’ Business Daily, the Financial Post, Reuters, National Post, Google News, Barron’s, China Daily, Huffington Post, AOL, City A.M. (London), Bloomberg, and the Independent (UK). He has also appeared in business interviews on the BBC, CBC, and SKY TV.