 In May, Stockhouse investors were alerted to a unique healthcare investment opportunity, centering on the world’s leading cause of permanent disability and one of the leading causes of death: stroke. That Stockhouse article spelled out the potential: The Revolutionary Healthcare Solution Potentially Worth $BILLIONS.
In May, Stockhouse investors were alerted to a unique healthcare investment opportunity, centering on the world’s leading cause of permanent disability and one of the leading causes of death: stroke. That Stockhouse article spelled out the potential: The Revolutionary Healthcare Solution Potentially Worth $BILLIONS.
The Company which has pioneered this healthcare solution is CVR Medical Corp (TSX: V.CVM, OTCQB: CRRVF, Forum). CVR Medical hasn’t found a cure for stroke, but what it has done is nearly as important. CVR has produced a safe, fast, repeatable, cost-effective technology for the early detection of the leading indicator of stroke.
Why is this so important? A stroke causes permanent damage. All that varies is the level/severity of damage to each individual to suffer a stroke. The only way to prevent such damage (and death) is to detect patients at high risk for stroke before the stroke occurs – and then seek preventative therapies for the patient to prevent a stroke event.
CVR Medical calls its ground-breaking innovation the “Carotid Stenotic Scan”. Where is the value in this proprietary healthcare technology?
Because it is safe, and because this diagnostic tool is at a price point lower than other existing modalities and does not require a certified technician to receive repeatable results, physicians will be less reluctant to implement the CSS.
Because it is fast, it provides the capacity (in the healthcare system) to monitor a much larger percentage of the population for risk of stroke.
Because it is cost-effective and easy-to-use, it would be affordable to monitor a much larger percentage of the population for risk of stroke.
What physician or hospital would not want to take advantage of such a revolutionary innovation?
Now CVR has commenced full-scale, clinical testing of the Carotid Stenotic Scan, with its research partner: Thomas Jefferson University. The first results of this clinical testing are now available. Stockhouse recently posed some questions to CVR’s CEO, Peter Bakema, to both interpret and explain these important results to the Stockhouse audience.
- For newer readers/investors, please introduce your Company and explain its roots.
- What started out as a theoretical conversation between a physicist and a mathematician has been transformed into a vessel poised to bring a game-changing technology to market, as CVR Global Inc. and CVR Medical Corp. have roots which stem back over 10 years. Built on a solid technology platform, CVR has the fundamentals and leadership to set it up for a strong market presence long into the future.
- How did the Carotid Stenotic Scan acquire its name?
- The Carotid Stenotic Scan’s (CSS) title is derived from the purpose of the device itself. It is a tool designed to collect sound data produced by the Carotid Arteries. It then analyzes this data, and though propriety software and hardware, is able to detect the level of stenosis (blockage) within the arteries.
- How does this diagnostic device work?
- The CSS listens to sounds produced by the flow of blood through the arterial system and analyzes the resulting output. By avoiding potentially dangerous contrast agents and radiation, the CSS offers a low risk tool for clinicians to identify arterial disease within the Carotid Arteries. This low level of risk, combined with the low price point and ease of use, positions the CSS as a weapon in the ever growing war on cardiovascular disease. By placing the tool in the early stage clinical care setting, such as the Primary Care Physician’s office, the clinician is able to detect the presence of disease and mitigate events such as stroke.
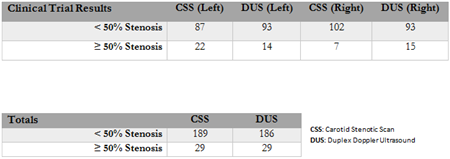
- On June 29, 2017; the Company issued a press release announcing initial results from the first clinical trials at Thomas Jefferson University (TJU). Could you provide the parameters of this study?
- In the study currently being conducted under the guidance of Dr. David Whellan, and his staff at Thomas Jefferson University Hospital, the CSS is being compared against other modalities for identifying the presence of disease within the Carotid Arteries.
- In its report on the clinical results, TJU was encouraged by the robust rate of patient enrollment in the study. To what did they credit the steady stream of enrollees?
- The success of patient enrollment at Thomas Jefferson University can be credited to several factors, the first being the level of professionalism and effectiveness put forth by the staff at the Jefferson Clinical Research Institute. Both Dr. Whellan, Suzanne Adams, and all the other team members have truly gone above and beyond to work with CVR. The second factor leading to a robust patient enrollment is the level of ease in which the CSS can be used. With a test taking less than two minutes, the process does not impede on the schedules of enrollees.
- What were the objectives of this initial clinical trial?
- The process of the clinical trials at Thomas Jefferson University Hospital is initially to validate and refine recent hardware and software improvements made to the CSS. Once this is confirmed then a second phase of clinical trials will be initiated in the near future. These pivotal trials will be used to compare the CSS to Duplex Doppler Ultrasound to confirm the safety and effectiveness of the device for submission to the FDA for market clearance in the United States.
- What results were reported by TJU in the June 29th press release?
- The Thomas Jefferson University Hospital report, which was included in the June 29th press release, goes to confirm the progress of the device within the clinical setting. The leadership at CVR could not be more pleased with the way the trial is being conducted and the way that the device is performing. Every aspect of the report coincides with our projected pathway and timeline to FDA submission.
- Looking further down the road, please expand upon the investment opportunity here if clinical testing continues to yield proof-of-concept results?
- As with any investment, product development, and general business venture, early stage investors accept a higher level of risk. We feel as though the progress, both clinically and operationally, goes to confirm the vision which many of our early stage investors have. The vision and belief that this project truly holds boundless potential and is not only poised to be fiscally beneficial the medical system, the patients, and to the shareholders of CVR, but more importantly to help save lives as the project rapidly advances.
FULL DISCLOSURE: CVR Medical Corp is a paid client of Stockhouse Publishing.