RE:RE:RE:RE:RE:Just three more weeks In my previous post, I forgot to mention "@120 days", as this was the whole point of the initial discussion. So it should have read like this:
The design of the clinical trial doesn't even include a meeting between the urologist and the patient @120 days. #########################################################################
It has nothing to do with how promising or not is a drug/treatment.
It simply has to do with clear endpoints specified in a clinical trial. That's how work all trials. And in this case, 90 days (CR) and 365 days (durable response) are the specific endpoints and this is the same for all NMIBC non-responsive trials as this is what is recommended by the FDA (go see the 2018 FDA guideline). Of course, in this specific trial, the 2nd treatment is @6-month. Of course, they have to do a cystoscopy. So they have the possibility to collect data unofficially.
The design of the clinical trial doesn't even include a meeting between the urologist and the patient @120 days.
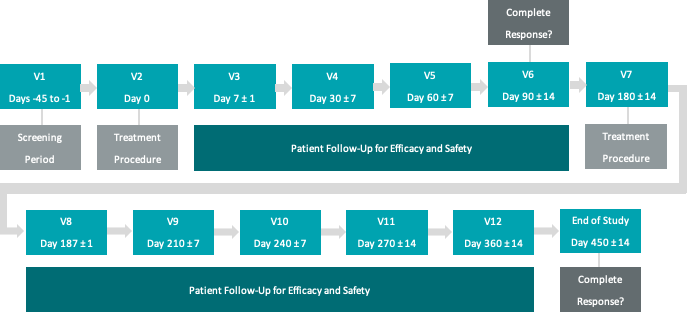
Not also this recent extension (this is the first time I notice that "450 days" mention), as I thougt it was at 360 days (12-months). It's rather 360+90:
Secondary endpoint of duration of CR at 360 days post-initial CR (approximately 450 days post initial Study treatment, assuming CR is achieved at the 90 day assessment)

versus: