RE:RE:Optimal Management of Bacillus Calmette-Guérin–Refractory NoThat was my thoughts as well , Compare latest results provided in quarterly , I am always confused now by the varying way resolts have been presented. I rely only on our stellar results posted recently.
Study II Preliminary Clinical Data:
To date, Study II has provided the primary study treatment for 63 patients.
Performance to Primary, Secondary and Tertiary Objectives
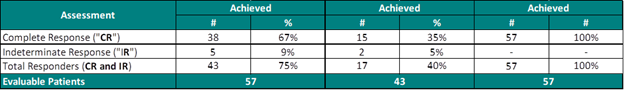
Study II Clinical Data Based on Assessment Visit
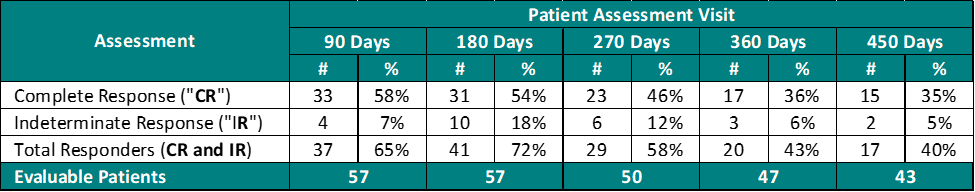
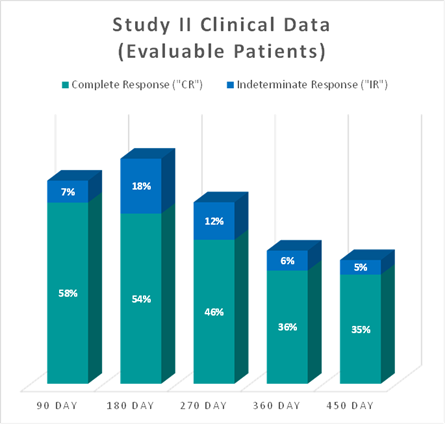
The interim clinical data demonstrates that at the 90 Day Assessment 58% of Evaluable Patients achieved a CR and 65% achieved a Total Response (CR + IR) post primary Study II Treatment and at 450 days 35% achieved a CR and 40% achieved a TR.
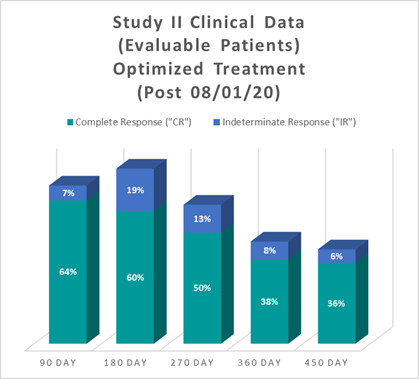
Study II Clinical Data Based on Assessment Visit for Patients Treated with the Optimized Study II Treatment (Post August 1, 2020)
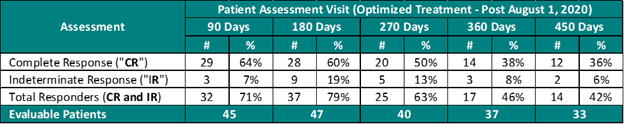
The interim clinical data demonstrates that at the 90 Day Assessment 64% of Evaluable Patients achieved a CR and 71% achieved a Total Response (CR + IR) post primary Study II Treatment with the Optimized Study II Treatment and at 450 days 36% achieved a CR and 42% achieved a TR.
Note:
- For patients to be included in the statistical clinical analysis they must be enrolled in Study II, provided the primary Study II Treatment and evaluated by a PI at the 90 day assessment visit (cystoscopy and urine cytology)
- One patient passed away prior to their 90 day assessment and is therefore not included in the statistical analysis; therefore, there are 63 patients that have been statistically analyzed.
- Evaluable Patients are defined as patients who have been evaluated by a PI and thus excludes a patient's clinical data at specific assessment days, if that clinical data is pending.
- Five patients have been enrolled and provided the primary Study II Treatment, but have not been evaluated at their 90 day assessment; therefore, 57 patients are considered Evaluable Patients at 90 days, with 43 patients considered Evaluable Patients at 450 days.
- 2023-11-29 | TSXV:TLT | Press Release | Theralase Technologies Inc (stockhouse.com)