MICRON Here's a micron size comparison.
Fokus lab assays used a 75 micron.
Fire assay.
Silica does not melt at 1000 degree ( fire assay )
If majority of gold is not visable at Galloway and host rock resembles greenstone,
high odds gold could be in a salt chloride form. ( solution )
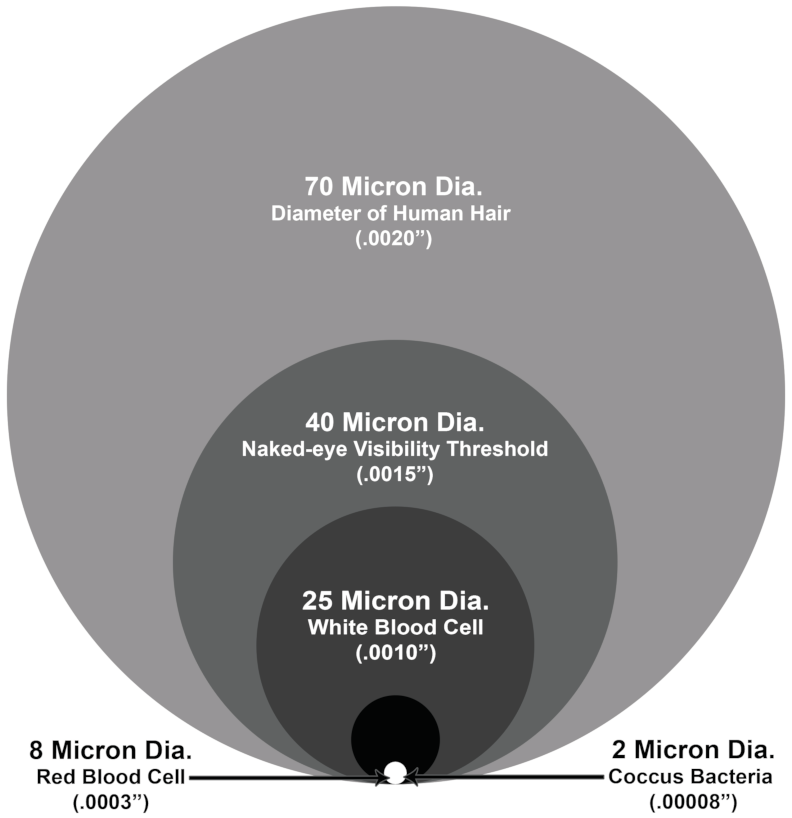
Imagine using a 40 micron size ( retest ores )
How much more gold could be present in a silicate / solution form ?
Hello.
It becomes even more interesting...
When one hones in on the aluminum.
Gold can form bonds with aluminum.
Galloway has good % of aluminum in it's ores but for some reason, avoids this topic.
chloroauric acid
[AuCl2]
Let's suppose Galloway's unseen gold is in salt chloride form.
What would a fire assay do to chloride gold if aluminum is also present and known to
form bonds with gold ?
Web Search
Gold–aluminium intermetallic is a type of intermetallic compound of gold and aluminium that usually forms at contacts between the two metals. Gold–aluminium intermetallic have different properties from the individual metals, such as low conductivity and high melting point depending on their composition.
Could significant chloric solution gold bond with aluminum during fire assay ?
I myself, would think the heavier element would drop out due to mass gravity but...
reading how gold can bond to aluminum and that there's ample aluminum at Galloway,
i would have to second guess my former assumptions.
I'd be running new 35/40 micron grind tests.
Pulling forward the aluminum to see if there exists natural gold aluminum bonds, or
if each might form bonds during fire assaying.
I'd focus on, full dissolution of minerals.
Then employ electrolysis to achieve a more accurate gold percentage/gram value.
Cheers...