Back in 2020
Roswell Park's
Drs. Gal Shafirstein &
Sarah Chamberlain were working with
Dr. McFarland on intra-operative photodynamic therapy for
NSCLC using
TLD1433 and
Lumeda's optical surface applicator. Ultimately, the use of
TLD1433 was abandoned in favour of
photofrin, which was invented at
Roswell Park) and two
IO-PDT clinical trials ensued.
Light Dosimetry for Photodynamic Therapy With Porfimer Sodium in Treating Participants With Malignant Mesothelioma or Non-Small Cell Lung Cancer With Pleural Disease Undergoing Surgery Photodynamic Therapy to Amplify the Response to Immunotherapy in Patients With Non-small Cell Lung Cancer With Pleural Disease These two trials could have used
TLD1433 as the photosensitizer and no information has leaked out about why
photofrin was preferred.
Now
Dr. Shafirstein has received a $1 million grant for a new project involving a new image-ibased
IO-PDT treatment of inoperable malignant tumours that obstruct airways.
 "Gal Shafirstein, DSc, Director of PDT Clinical Research, and Nathaniel Ivanick, MD, Assistant Professor of Oncology in the Department of Thoracic Surgery, received a two-year $1 million grant from the NCI, with support from Simphotek Inc., to study a new image-based treatment for interstitial photodynamic therapy (I-PDT) of inoperable malignant tumors with airway obstruction. “During I-PDT in the clinic, there is a need to adjust the irradiance (light dose rate) and fluence (light dose) to account for patient-specific tissue and tumor optical properties or changes in fiber placement that occur after an initial pretreatment plan is generated,” says Dr. Shafirstein, the co-Principal Investigator. “This can be accomplished with novel, near-real-time computational software that will be employed in this phase 2a trial to calculate the intratumoral fluence and irradiance, which will impact tumor response in I-PDT of locally advanced cancers.” Innovative Proposals From Roswell Park Teams Draw More Than $36 Million in Research Grants Drs. Shafirstein
"Gal Shafirstein, DSc, Director of PDT Clinical Research, and Nathaniel Ivanick, MD, Assistant Professor of Oncology in the Department of Thoracic Surgery, received a two-year $1 million grant from the NCI, with support from Simphotek Inc., to study a new image-based treatment for interstitial photodynamic therapy (I-PDT) of inoperable malignant tumors with airway obstruction. “During I-PDT in the clinic, there is a need to adjust the irradiance (light dose rate) and fluence (light dose) to account for patient-specific tissue and tumor optical properties or changes in fiber placement that occur after an initial pretreatment plan is generated,” says Dr. Shafirstein, the co-Principal Investigator. “This can be accomplished with novel, near-real-time computational software that will be employed in this phase 2a trial to calculate the intratumoral fluence and irradiance, which will impact tumor response in I-PDT of locally advanced cancers.” Innovative Proposals From Roswell Park Teams Draw More Than $36 Million in Research Grants Drs. Shafirstein and
Ivanick will be working with a very interesting company called
Simphotek Inc., which specializes in devices to facilitate treatment planning and dosimetry for light based cancer treatments. They are currently involved in 2 clinical trials using
photofrin. They could just as easily be using
TLD1433 and may have gotten a much better result. It would take someone smarter and better informed than me to figure out if this is another missed opportunity or is sound business strategy on the part of
Theralase.
NEWARK-BASED SIMPHOTEK HELPS SURGEONS PRECISELY TARGET TUMORS THROUGH REAL-TIME DIGITAL VISUALIZATION September 30, 2020 Esther Surden
Suppose there is a therapy in development that could treat your internal solid tumors and stimulate your immune system to significantly cut the risk of your cancer returning?
What if this therapy, already in introductory clinical trials, spared your organs, so you could keep your tongue or your lung?
What if it did all this, but also used technology that wouldn’t damage your DNA?
Simphotek, a Newark-based biotech company, has developed a digital technology that guides surgeons as they use photodynamic therapy to remove solid tumors caused by advanced cancer.
Simphotek was recently selected as a “showcase company” at the National Cancer Institute’s Small Business Innovation Research (NCI SBIR) virtual event on Sept. 9–10, and as a “breakthrough company” at the international Redefining Early Stage Investments (RESI) conference on Sept. 14–16. Additionally, on Sept. 21 Simphotek presented its technology as one of the top 20 biotech companies in cancer therapy at an international meeting. 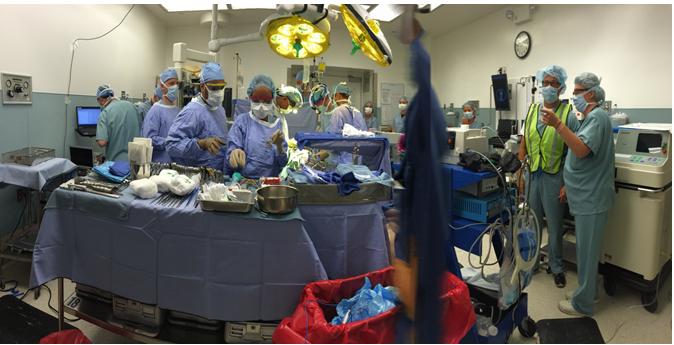
Simphotek in the treatment room | Courtesy Simphotek Simphotek uses computer hardware and software, including patent-pending algorithms, to create a treatment-planning system called “Dosie,” which works in real time inside the treatment room. The system depends on a photoreactive drug that binds with cancer cells. The software calculates how many cancer-killing ions a surgeon needs, the right amount of light intensity, how long the surgeon will need that light intensity and the right amount of the drug to use.
“In addition to doing these calculations, we have developed a combination of computer processors so that the calculations that the surgeon needs are determined in a few seconds, rather than the hours that usually take place for this type of calculation,” Mary Potasek, cofounder and chief science officer, told us.
Simphotek was founded in 2016 by Potasek; Gene (Evgueni) Parilov, CTO; and Karl Beeson, vice president of technology.
“The program can calculate changes that the surgeon will need, using the input parameters, the light intensity, the light duration, and so on, while a surgeon is actually operating,” Potasek said. It can tell the surgeon, “‘You need such-and-such light intensity, but you do not have enough and, in order to create enough cancer-killing ions, you need to increase that amount.’ And that calculation can be done in a few seconds. This is a complicated calculation that normally takes hours to process,” she added.
“We have a combination of various computer processors — The graphics processing unit and CPU — that we’ve added to make the cancer therapy reproducible, which has never been done before for this type of therapy. This is a critical unmet need to advance this cancer technology,” Potasek told us.
“We also give the surgeons a three-dimensional map of the tumor,” she said, “so, they can look inside the tumor at various points and see which regions, down to a millimeter, are not receiving enough cytotoxic agents. And that region may have to be a treated longer or may have to have the light intensity increased or decreased.”
For patients, besides the promise of a longer life, the treatment would be highly sought after because it doesn’t have many negative side effects, such as impotence, nausea, organ removal or damage or DNA damage,” Potasek stated.
Simphotek has raised about $7 million since 2016. Most of the money came from National Cancer Institute grants. The company collaborates with two world-leading medical schools — University of Pennsylvania and the Roswell Park Comprehensive Cancer Center — to form a multidisciplinary group of over 20 surgeons, doctors, engineers, biologists, radiation physicists and oncologists, all working together with one goal: to enable physicians to use photodynamic therapy to double or triple patient life expectancy when treating solid tumor cancers.
Currently the company is proposing to raise $3.5 million to finalize intellectual property, hire key management, and develop a working prototype. The company estimates that it will later have to raise about $20 million to complete development and do clinical trials, Potasek said. Simphotek Medical Devices