 (Image via @revivetherapeuticsltd on Instagram.)
Psychedelics, like psilocybin are quickly becoming mainstream. In the past few months, its use has been legalized in Denver, Oakland, and Santa Cruz, while lawmakers in Ann Arbor, Michigan have voted to decriminalize its consumption and it is on the ballot in Oregon to be legalized in November.
(Image via @revivetherapeuticsltd on Instagram.)
Psychedelics, like psilocybin are quickly becoming mainstream. In the past few months, its use has been legalized in Denver, Oakland, and Santa Cruz, while lawmakers in Ann Arbor, Michigan have voted to decriminalize its consumption and it is on the ballot in Oregon to be legalized in November.
The key to treatments for a host of mental issues is almost ready to be unlocked. From obsessive-compulsive disorder, post-

traumatic stress disorder, opioid addiction, alcoholism, depression, anxiety, and eating disorders, numerous companies are stepping up clinical trials supporting psychedelic treatments, and one publicly traded Canadian Company is getting its clinical trials backed by a US university.
Focused on the research and development of therapeutics for medical needs and rare disorders is
Revive Therapeutics Ltd. (CSE: RVV, Forum).
The specialty life sciences Company provided investors with an update on its psychedelics therapeutics programs specifically as it relates to the Company’s oral thin-film delivery system and clinical studies with psilocybin at the University of Wisconsin-Madison.
The Company’s Chief Executive Officer, Michael Frank explained in a
September 2020 news release that RVV is expanding its product pipeline with a focus on psychedelic therapeutics, by incorporating its novel oral thin-film delivery technology with psilocybin.
The team has developed prototypes and will move towards clinical studies with the University of Wisconsin-Madison along with other key industry partners.
“In addition, we are advancing our Phase I clinical study to evaluate the safety and feasibility of psilocybin in adults with Methamphetamine Use Disorder. Our initiatives in product development and clinical studies gives us a leading position in the psychedelic space.”
“Phase I Study of the Safety and Feasibility of Psilocybin in Adults with Methamphetamine Use Disorder:”
This clinical trial study of psilocybin to treat Methamphetamine Use Disorder among adults was recently announced by the Company as an agreement with the Board of Regents of the University of Wisconsin System.
The University’s School of Medicine and Public Health will conduct the clinical study, along with the School of Pharmacy, which holds a Wisconsin special authorization and DEA license to perform clinical research with psilocybin. Key intellectual property from this study will belong exclusively to Revive Therapeutics.
The Principal Investigator for this Phase I study is Dr. Christopher R. Nicholas, Ph.D. He is an Assistant Professor of Program for Research Outreach Therapeutics and Education in the Addictions in the Department of Family Medicine and Community Health at the University’s School of Medicine and Public Health.
Psilocybin oral thin-film product:
The University of Wisconsin-Madison, through the Reed Research Group is also helping sponsor a research partnership with Revive Therapeutics to develop its tannin-chitosan composite of orally dissolvable thin films. This offers a rather unique delivery platform for therapeutic doses (1-20mg) of psilocybin into the oral cavity.
The team at Revive has just received its final set of prototypes and is preparing to scale for manufacturing for future clinical studies, which will involve psilocybin and other psychedelic-derived medicines. Psilocybin thin film that dissolves orally offers several advantages and benefits, such as:
- Dissolves rapidly
- Onset of action to the bloodstream,
- Ease and convenience for patients to administer without the need of water, chewing or swallowing
- Potential of improved therapeutic outcomes and efficacy for underserved diseases and disorders including the flexibility to create accurate dosing and tasteful options
This key delivery technology has a rapid onset of action and controlled, or sustained, release potential capabilities which may allow combining multiple extracts from mushrooms in one formulation.
This technology is also made from a natural, non-toxic, biodegradable and biocompatible composite that combines a tannin material (derived from a plant group having antibacterial, antifungal, antioxidant and wound healing properties), and a chitosan material, (derived from the crustacean group having blood-clotting and antimicrobial properties).
Investment conclusion:
Revive Therapeutics is already ahead of the game with its research and development of psilocybin and the day is fast approaching when major pharmaceutical companies will invest heavily into this space, and incorporate psychedelics into their own drug pipelines.
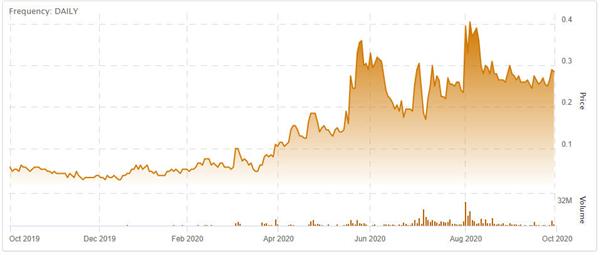 (Revive Therapeutics stock chart, Sept 2019 – Sept 2020. Click to enlarge.)
(Revive Therapeutics stock chart, Sept 2019 – Sept 2020. Click to enlarge.)
Company shares have performed well in recent months after a shift in focus to temporarily aid in the fight against COVID-19. Revive Therapeutics is looking to join the fight directly, having already received FDA approval for Phase III trials
evaluating the use of Bucillamine for treatment of COVID-19 patients.
For more information on this Company, visit
www.ReviveThera.com.
FULL DISCLOSURE: This is a paid article produced by Stockhouse Publishing.