 (Image via Pharmather Inc.)
An undervalued public Company in the psychedelic space is targeting unmet medical needs via psychedelics, de-risking a pathway to clinical trials and partnerships.
(Image via Pharmather Inc.)
An undervalued public Company in the psychedelic space is targeting unmet medical needs via psychedelics, de-risking a pathway to clinical trials and partnerships.

Biotech pharmaceutical Company
PharmaTher Inc. (CSE: PHRM, Forum), a subsidiary of Newscope Capital Corporation, is focused on repurposing and finding new or novel formulations of psychedelics for the disorders of the brain and nervous system. The Company is focusing more on the clinical development of ketamine through the US Food and Drug Administration’s (FDA) regulatory approval process.
The intent is to make its products available by prescription, much like what
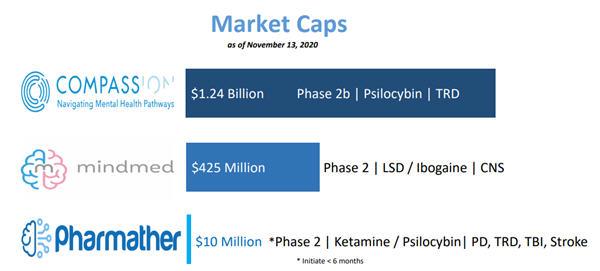
COMPASS Pathways (NASDAQ: CMPS) is doing with psylocibin or
MindMed (NEO: MMED, OTCQB: MMEDF) with LSD.
Yet PharmaTher (a hybrid of “pharma” and “therapeutics”) takes the position where it looks at drugs like ketamine, an original in the psychedelics space with an FDA approved product, and the team looks to find new uses for it, as well as new formulations and how to deliver them a different way.
PharmaTher has three kinds of technology platforms:
- Discovery - Artificial Intelligence (AI) for new uses of psychedelics and combinations with FDA drugs via its proprietary tech platform – panaceAI™
- Development - R&D in combining psychedelics with FDA approved drugs
- Delivery - Novel microneedle delivery patch for psychedelics
Let’s take a deeper look into each of these platforms and how they allow PharmaTher to take full advantage of this $100 billion (USD) market potential.
panaceAI
™ is a proprietary drug repurposing platform, which focuses on the psychedelics that target specific receptors in the brain and look for these types of new uses and combinations with other FDA-approved drugs to eliminate the side effects that the psychedelic drugs have (hallucinations, amnesia). The Company wants to make an improvement to these psychedelics with FDA-approved drugs so that it can move faster to clinical trials and then faster to market as typical new drug development.
Through the Company’s drug development platform, the team looks to repurpose these compounds very quickly into clinical trials so that they can be validated for human use.
From there, the Company can license its drugs to big pharma companies or submit to market approval through regulatory channels. And then within that, the team looks for the uniqueness of these psychedelics to deliver them in a different way.
The Company found that its licensed microneedle delivery system offers a dramatic improvement to the current way of being of psychedelics being delivered, whether it's by oral or injectable. Like a transdermal patch, this microneedle can efficiently deliver these drugs into the body quickly and control the way how it is delivered.
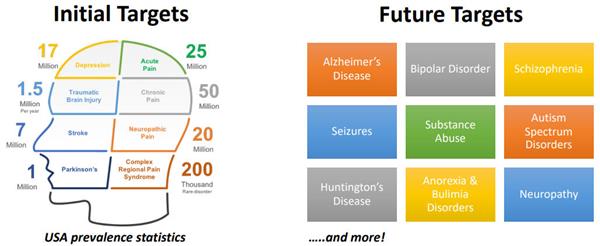
As PharmaTher investigates new opportunities in psychedelics and works to reduce the risk, cost, and development time toward FDA approval, whether it is for the FDA’s 505(b)(2) regulatory pathway, the Company also looks to rare diseases and has an Orphan Drug Designation (ODD) specifically applied for Parkinson’s disease.
There is a vast pipeline, mainly with ketamine, for Parkinson’s disease, to reduce or eliminate dyskinesia, or movement disorders. Case studies have found that when ketamine was used to treat Parkinson’s patients for their depression and pain, these Parkinson’s patients with dyskinesia saw their conditions subside for more than 30 days, in one patient this relief lasted for three months.
Upon this discovery, patents were filed and PharmaTher owns the use of ketamine for Parkinson's disease, and now it is going to be moving it towards a Phase 2 clinical early next year and is in the process of filing its investigational new drug (IND) application with the FDA.
 (Image via PharmaTher Inc.)
(Image via PharmaTher Inc.)
PharmaTher boasts a robust IP portfolio that sets itself apart from other psychedelic companies in this space.
With its patents for new reformulated versions of ketamine, it's more of an advanced clinical stage pipeline from psychedelics targeting significant unmet clinical needs.
In October 2020, the Company filed an application with the FDA to receive an Orphan Drug Designation (ODD) for ketamine in the treatment of Postherpetic neuralgia (PHN), a chronic neuropathic pain syndrome resulting from an outbreak of the herpes zoster virus, also known as shingles.
The Company’s Chief Executive Officer, Fabio Chianelli stated in
a news release that this FDA orphan drug application for ketamine to treat PHN is complementary to its growing psychedelic drug development pipeline for rare disorders. The global PHN market is expected to be valued at USD $908.4 million by 2026, according to Persistence Market Research.
“We are focused on building a unique ketamine franchise targeting unmet medical needs such as Parkinson’s disease, depression and pain. We are leveraging scientific and clinical data to advance the development of ketamine through the FDA approval process, as well as carving our niche in the rapidly evolving psychedelic pharmaceuticals market.”
PharmaTher doubled-down on its discovery platform when it announced in
November 2020 that it had filed a provisional patent application with the US Patent and Trademark Office outlining the potential novel use of psilocybin to treat cancer, which was also discovered by panaceAI. The next move for the Company is to advance the R&D of psilocybin for cancer with leading US research institutions with the objective to complete investigational new drug (IND)-enabling studies and to file an IND application with the US Food and Drug Administration.
CEO Chianelli pointed to this as an example of how the Company is broadening its psychedelic-based patent portfolio and build our psilocybin product pipeline in neurological (traumatic brain injury and stroke) and cancer disorders that have the potential to obtain FDA orphan drug designation. The Company is seeking to partner their psilocybin program to focus on its lucrative ketamine programs and commercialization of panaceAI
™.
“Although we have positioned ourselves as a specialty pharmaceutical company advancing the clinical development of ketamine to treat Parkinson’s disease, depression and pain, we continue to leverage our drug repurposing artificial intelligence platform, panaceAI
™, to discover new uses of psychedelics that we may develop internally or partner with life sciences companies. In addition, we are exploring novel uses of psychedelic drugs such as Ketamine, LSD, MDMA, Ayahuasca and combinations with FDA approved drugs to improve on their efficacy and side effects profile for clinical development and business development opportunities.”
This discovery proves what a unique and valuable asset the panaceAI
™ platform is for PharmaTher. As CEO Chianelli explained, the AI tool collects all the information available around diseases and then the team can use its algorithm to find new uses for ketamine.
In an interview with Stockhouse Editorial, he stated, “What we found was that as we building these algorithms to allow to kind of reduce the bias of this data to extrapolate so that it could provide an inventive step to find even new uses that probably wouldn’t be to the eye of the beholder. What’s unique about our panaceAI
™, is that we are combining these different elements. Not only from the algorithms from these models that we built but also looking at certain clusters like cluster analysis and docking analysis, the formats to allow for these predictions.”
From there, the team engages in virtual pre-screening, so that they can determine the next steps without investing real money, in terms of doing the laboratory in vitro models, but they can show in a virtual way that these are predictions that are validated and can move into preclinical models to really understand the proper dosing.
Understanding dosing is what is especially unique about this panaceAI
™, is that because it looks at other FDA approved compounds to show that if they have some complementary or synergistic effects, it created a combination type drug program, so that it can have a super version or a “psychedelic 2.0 compound”. This can lead to a therapeutic benefit for the 260+ million global patients living with depression who could turn to this treatment but would not be susceptible to known side-effects like hallucinations.
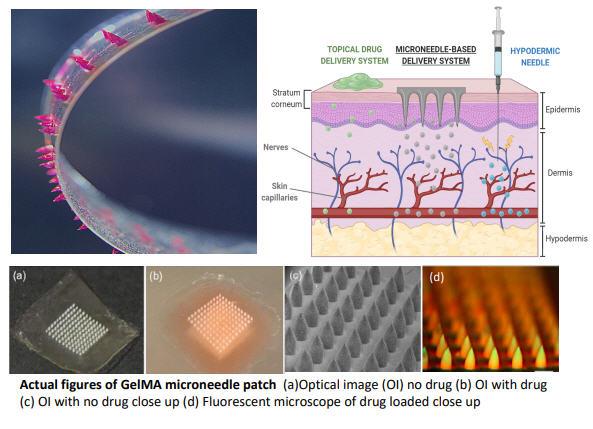
The other cutting-edge delivery platform PharmaTher offers is its microneedle patch, which the Company has licensed. Created out of a UCLA laboratory, these microneedles do not harm the skin and it is safe. They are biodegradable and bio dissolvable, while allowing for efficient penetration of the drug to enter past the top layer of the skin. It can also be developed to be placed on the inner cheek walls of the mouth or under a patient’s tongue for fast delivery and can use a combination of drugs, whereas a thin film strip is much more limited in its loading capacity.
 (Image via PharmaTher Inc.)
(Image via PharmaTher Inc.)
For more on the microneedle, visit this
October 2020 news release, where CEO Chianelli stated that, upon acquiring the rights to this novel microneedle delivery technology, it strengthens the foundation of the Company’s strategy to develop and commercialize a unique pipeline of psychedelic pharmaceuticals for FDA approval.
He believes it puts PharmaTher in the conversation with companies such as Compass Pathways and Mind Medicine, who are leading the way in psychedelic medicines.
“The GelMA-MN delivery technology is complementary to our approach in finding new uses and combinations of psychedelics to improve therapeutic and safety outcomes while potentially offering a differentiated product profile, improving patient compliance and enabling out-patient treatment options. We are focused on realizing the potential of the GelMA-MN delivery technology and it will open up new market opportunities in multi-billion dollar categories such as mental health, nervous system disorders, pain, skin cancer, wounds, mucosal diseases and surgical applications.”
 (Image via PharmaTher Inc.)
(Image via PharmaTher Inc.)
CEO Chianelli has been in the biotech sector for nearly 20 years and has focused on earlier clinical stage companies and the drug delivery and drug repurposing space. It is experience that is shared among the Company’s board of directors. New to the team is
Dr. Robert Hauser is a very well-known leader in Parkinson's disease. He was a clinical investigator for over a hundred clinical studies in Parkinson’s, along with prolific Parkinson’s Researcher,
Dr. Alberto J. Espay, MD, MSc, FAAN, who joined PharmaTher in November as a scientific and clinical advisor.
 (Image via PharmaTher Inc.)
(Image via PharmaTher Inc.)
PharmaTher Inc. is a pure-play biotech pharmaceutical psychedelic Company with a reasonable valuation compared to other psychedelic companies and has shown that its drug has been proven to work in certain indications now going into clinical trials. This is an opportunity for investors to get on the ground floor of a psychedelic biotech Company.
Investors should keep track of this Company to see what develops and to find out more, visit
pharmather.com.
FULL DISCLOSURE: This is a paid article produced by Stockhouse Publishing.