Updated results in seven patients with advanced uveal melanoma treated with CHEMOSAT and ipilimumab plus nivolumab show a Median PFS of 29.1 months
All seven patients treated still alive as of last follow-up at a median follow-up of 29.1 months
NEW YORK, Jan. 17, 2023 /PRNewswire/ -- Delcath Systems, Inc. (Nasdaq: DCTH), an interventional oncology company focused on the treatment of primary and metastatic cancers of the liver, today announced the publication of updated results from the Phase 1b CHOPIN Trial, conducted at Leiden University Medical Center on the use of the Delcath CHEMOSAT® Hepatic Delivery System with Melphalan (CHEMOSAT) in combination with the immune checkpoint inhibitors (ICI) ipilimumab and nivolumab to treat patients with metastatic uveal melanoma with liver metastases. The publication is entitled "Combining Melphalan Percutaneous Hepatic Perfusion with Ipilimumab Plus Nivolumab in Advanced Uveal Melanoma: First Safety and Efficacy Data from the Phase 1b Part of the CHOPIN Trial" and was published in Cardiovascular and Interventional Radiology.
Updated CHOPIN Phase 1b Trial Results
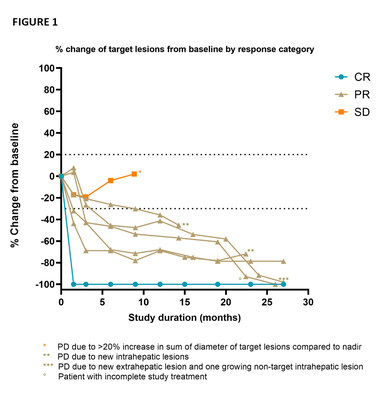
The goal of the CHOPIN trial is to study the safety and potential synergistic effects of systemic ICI therapy ipilimumab plus nivolumab (IPI+NIVO) when combined with Delcath's proprietary liver-targeted percutaneous hepatic perfusion (PHP) treatment in metastatic uveal melanoma patients. The just released publication presented updated safety and efficacy results from the Phase 1b portion of the trial which were previously presented in June 2022 at the American Society of Clinical Oncology Annual Meeting. The Phase 1b portion of the trial enrolled seven patients each of which were treated with two courses of PHP (melphalan 3mg/kg, max 220 mg per cycle) combined with four courses IPI+NIVO escalating the dosing from 1mg/kg each IPI+NIVO (cohort 1) to IPI 1mg/kg + NIVO 3mg/kg (cohort 2). As previously reported, the Best Overall Response included 1 complete response, 5 partial responses and 1 stable disease accounting for an Objective Response Rate of 85.7% and a Disease Control Rate of 100% (FIGURE 1). At the cut-off date of November 15, 2022, the median follow-up was 29.1 months (range 8.9 – 30.2), the median PFS was 29.1 months (95% CI 11.9 – 46.3) and the median duration of response was 27.1 months (range 7.4 – 28.5). All patients are still alive and three of four patients who subsequently experienced PD continued with treatment in the form of repeated melphalan PHP (M-PHP) cycles.
The ongoing randomized phase 2 part of the CHOPIN trial comparing M-PHP alone with M-PHP plus IPI/NIVO, which will include another 76 patients (38 per arm), is approximately 50% enrolled and will provide more insight to the efficacy.
The determination of a safe and effective dose was a primary goal of the Phase 1b portion of the CHOPIN trial. Grade 1/2 adverse events were seen in all patients and 71.4% experienced grade 3/4 toxicities. In this phase 1b dose-escalation study combining M-PHP with IPI/NIVO the safe treatment dose was established at IPI 1mg/kg and NIVO 3mg/kg. The authors did observe low-grade immune-related toxicities and PHP-related hematological toxicities in the treated groups. Hematological toxicity is a common adverse event after M-PHP, affecting approximately three-quarters of patients. All 7 patients in the study experienced grade 1/2 anemia. To prevent severe leukopenia/neutropenia, G-CSF was administered within 48 hours after M-PHP in their treatment center. The phase 2 part of the CHOPIN study will provide more information on both hepatic and systemic toxicity associated with the combination therapy.
"If similar results are observed in the larger, randomized second phase of this trial, it would represent a meaningful improvement over current treatment options for this patient population," said Dr. Johnny John, Senior Vice President, Medical and Clinical Affairs. "In addition, further investigation of this combination protocol may be warranted in patients with liver dominant disease in other tumor types currently treated with ICI agents."
Rationale for Combination Therapy
The rationale for combining ICI therapy with M-PHP in metastatic uveal melanoma is based on both uveal melanoma's specific characteristics and the unique immunomodulatory role of the liver. Uveal melanoma is characterized by a different set of driver mutations and lower mutational load compared to cutaneous melanoma, leading to limited neoantigen presentation and lower efficacy of ICI (1). By combining M-PHP with IPI/NIVO, the authors noted that they aim to increase the efficacy of ICI by turning a 'cold tumor' into a 'hot tumor'. In addition, while PHP can provide long-lasting disease control in the liver, it does not control extrahepatic disease. Conversely, IPI/NIVO treatment shows a trend towards control of extrahepatic lesions, but hepatic disease progression regularly occurs (2, 3, 4). With combined treatment, the authors aim to control hepatic disease, as well as prevent extrahepatic disease in follow-up. In addition, it is well documented that the liver has a unique immune modulating role and liver metastases diminish ICI efficacy systemically regardless of the type of primary tumor (5, 6, 7) and in animal models it has been shown that this effect can be overcome by localized hepatic therapy (8). Current available evidence from studies on isolated limb perfusion (ILP) and isolated hepatic perfusion (IHP), which is the surgical counterpart of M-PHP, show that ILP and IHP can lead to T-cell activation following the procedures (9, 10). The authors hypothesize that this is also the case for M-PHP leading to an improved activation of the immune system together with ICI.
A PDF of the article abstract can be viewed by clicking here.
About Delcath Systems, Inc.
Delcath Systems, Inc. is an interventional oncology company focused on the treatment of primary and metastatic liver cancers. The company's proprietary percutaneous hepatic perfusion (PHP) system is designed to administer high-dose chemotherapy to the liver while controlling systemic exposure and associated side effects. In the United States, the PHP system is being developed under the tradename HEPZATO™ KIT (melphalan hydrochloride for injection/Hepatic Delivery System), or HEPZATO, for the treatment of patients with unresectable hepatic-dominant metastatic ocular melanoma (mOM), also known as metastatic uveal melanoma (mUM) and is considered a combination drug and device product regulated as a drug by the United States Food and Drug Administration (FDA).
In Europe, the PHP system is now regulated as a Class lll medical device and is approved for sale under the trade name CHEMOSAT Hepatic Delivery System for Melphalan, or CHEMOSAT, where it has been used at major medical centers to treat a wide range of cancers of the liver.
Safe Harbor / Forward-Looking Statements
The Private Securities Litigation Reform Act of 1995 provides a safe harbor for forward-looking statements made by the Company or on its behalf. This news release contains forward-looking statements, which are subject to certain risks and uncertainties that can cause actual results to differ materially from those described. Factors that may cause such differences include, but are not limited to, uncertainties relating to: the timing and results of the Company's clinical trials, our determination whether to continue a clinical trial program or to focus on other alternative indications, and the impact of the COVID-19 pandemic on the completion of our clinical trials; the impact of the presentations at major medical conferences and future clinical results consistent with the data presented; the Company's ability to successfully commercialize the HEPZATO KIT/CHEMOSAT system and the potential of the HEPZATO KIT/CHEMOSAT system as a treatment for patients with primary and metastatic disease in the liver; our ability to obtain reimbursement for commercialized product in various markets; actions by the FDA or foreign regulatory agencies; the Company's ability to successfully enter into strategic partnership and distribution arrangements and the timing and revenue, if any, of the same; uncertainties relating to the timing and results of research and development projects; and uncertainties regarding the Company's ability to obtain financial and other resources for any research, development, clinical trials and commercialization activities. These factors, and others, are discussed from time to time in our filings with the Securities and Exchange Commission. You should not place undue reliance on these forward-looking statements, which speak only as of the date they are made. We undertake no obligation to publicly update or revise these forward-looking statements to reflect events or circumstances after the date they are made.
Contact:
Investor Relations Contact:
Ben Shamsian
Lytham Partners
646-829-9701
shamsian@lythampartners.com
References
- Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005;123(12):1639-43. https://doi.org/10.1001/archopht.123.12.1639.
- Pelster MS, Gruschkus SK, Bassett R, Gombos DS, Shephard M, Posada L, et al. Nivolumab and Ipilimumab in Metastatic Uveal Melanoma: Results From a Single-Arm Phase II Study. J Clin Oncol. 2021;39(6):599-607. https://ascopubs.org/doi/full/10.1200/JCO.20.03274
- Karydis I, Chan PY, Wheater M, Arriola E, Szlosarek PW, Ottensmeier CH. Clinical activity and safety of Pembrolizumab in Ipilimumab pre-treated patients with uveal melanoma. Oncoimmunology. 2016;5(5):e1143997. https://doi.org/10.1080/2162402X.2016.1143997
- Rozeman EA, Prevoo W, Meier MAJ, Sikorska K, Van TM, van de Wiel BA, et al. Phase Ib/II trial testing combined radiofrequency ablation and ipilimumab in uveal melanoma (SECIRA-UM). Melanoma Res. 2020;30(3):252-60. https://doi.org/10.1097/CMR.0000000000000653
- Xiao-Juan C, Aiqun R, Liang Z, En-Dian Z, Tao J. Pan-Cancer Analysis Identifies Liver Metastases as Negative Predictive Factor for Immune Checkpoint Inhibitors Treatment Outcome. Frontiers in Immunology 12, (2021). https://www.frontiersin.org/articles/10.3389/fimmu.2021.651086
- Lee J, Mehdizadeh S, Smith J, Young A, Mufazalov I, Mowery C, et al. Regulatory T cell control of systemic immunity and immunotherapy response in liver metastasis. ScienceImmunology. 2020;5(52). https://doi.org/10.1126/sciimmunol.aba0759
- Yu, J., Green, M.D., Li, S. et al. Liver metastasis restrains immunotherapy efficacy via macrophage-mediated T cell elimination. Nat Med 27, 152–164 (2021). https://doi.org/10.1038/s41591-020-1131-x
- Xin, B, Yang, M, Wu, P, Du, L, Deng, X, Hui, E, et al. Enhancing the therapeutic efficacy of programmed death ligand 1 antibody for metastasized liver cancer by overcoming hepatic immunotolerance in mice. Hepatology. 2022; 76: 630– 645. doi:10.1002/hep.32266.
- Johansson J, Kiffin R, Andersson A, Lindnér P, Naredi PL, Olofsson Bagge R, et al. Isolated Limb Perfusion With Melphalan Triggers Immune Activation in Melanoma Patients. Frontiers in Oncology. 2018;8:570. https://doi.org/10.3389/fonc.2018.00570
- Johansson J, Siarov J, Kiffin R, Molne J, Mattsson J, Naredi P, et al. Presence of tumor-infiltrating CD8(+) T cells and macrophages correlates to longer overall survival in patients undergoing isolated hepatic perfusion for uveal melanoma liver metastasis. Oncoimmunology. 2020;9(1):1854519. https://doi.org/10.1080/2162402X.2020.1854519

 View original content to download multimedia:https://www.prnewswire.com/news-releases/delcath-systems-announces-updated-results-from-chopin-phase-1b-trial-published-in-cardiovascular-and-interventional-radiology-301722910.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/delcath-systems-announces-updated-results-from-chopin-phase-1b-trial-published-in-cardiovascular-and-interventional-radiology-301722910.html
SOURCE Delcath Systems, Inc.
