Visit booth 307 to learn how Covalon is creating a new standard for compassionate IV care technology designed to help protect patients from infections, pain, and trauma
Covalon Technologies Ltd. (the "Company" or "Covalon") (TSXV: COV; OTCQX: CVALF), an advanced medical technologies company, today announced its participation in the 2023 Annual Oley Foundation Conference being held in St. Louis, Missouri from Tuesday, June 27 to Friday, June 30, 2023. Patients, caregivers, healthcare providers, and advocates looking for more compassionate care solutions for those living with intravenous therapies should visit Covalon at booth 307 to learn more about Covalon’s unique position to provide “apology-free” dressings that are gentle on skin.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230627972227/en/
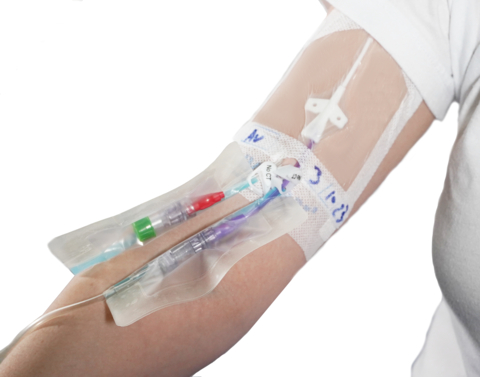
IV fluid line secured to the forearm with IV Clear®, a soft dual-antimicrobial silicone IV securement dressing offered by Covalon. (Photo: Business Wire)
“There is no reason why patients living with daily or frequent IV-line care need to live with the pain, discomfort, and worry that can come with receiving IV therapy,” said Ron Hebert, Senior Vice President, Marketing, Covalon. “For those relying on parenteral nutrition, their IV is a lifeline, but it can also be a direct line to infection, pain, and trauma. At Covalon, we’re committed to improving the patient experience with comfortable and flexible dressings and vascular access line guards that help protect the line, while providing patients with the flexibility and freedom they deserve.”
Stable and safe intravenous access with a central venous catheter is an absolute requirement for delivery of total parenteral nutrition (TPN). Dressings and line guards play a critical role in protecting patients from an increased risk of infection that comes with TPN (long term and sometimes for life) and keeping the vulnerable skin surrounding line sites healthy and comfortable.
Not all IV dressings are made equal. Due to differences in adhesive materials and active ingredients, IV dressings may not provide patients the protection from infection intended and cause medical adhesive related skin injuries (MARSI) surrounding the IV insertion site, an area vulnerable to pathogen entry.
The two most common adhesive components used in IV dressings are acrylic and silicone:
- Acrylic adhesives are known to be incredibly strong due to their tackiness. Acrylic can perform well when it comes to holding, fixing, and gripping - but it can be incredibly traumatic to the patient’s skin. Acrylic can also be time consuming to move, requiring healthcare providers to use solvents and scrapers.
- Soft silicone dressings are tacky enough to adhere to the surrounding skin, while being gentle enough to allow for minimized trauma during dressing changing.
If a patient has delicate skin and will be wearing an IV securement dressing for long periods of time, a silicone dressing is best for minimizing pain and trauma.
Covalon’s IV Clear dressing is the world’s only dual antimicrobial silicone adhesive vascular access dressing. Its features include:
- Chlorhexidine and silver embedded into the entire surface of the dressing provide broad spectrum protection.
- Silicone adhesive helps to maintain skin integrity and keeps patients comfortable.
- Complete transparency allows for visualization and easy daily assessment of the site and surrounding skin.
Covalon’s other IV-related solutions include:
- CovaClear® IV – utilizes soft silicone adhesive technology to help protect patients from skin injuries, but does not incorporate antimicrobials, for use with patients who either don't require or cannot tolerate antimicrobials.
- VALGuard® - a transparent, environmental barrier designed to protect catheter hubs and line connections from external contaminants and gross contamination, including body fluids and other secretions. It incorporates a quick-release pull strip for fast access to infusion hubs and for easy removal.
The 2023 Annual Oley Foundation Conference
The Oley Foundation aims to enrich the lives of those living with home intravenous (IV) nutrition or tube feeding through advocacy, education, community, and innovation. The conference brings together clinicians, patients, and industry to facilitate knowledge sharing on tube feeding and IV nutrition. Covalon representatives will be available to meet with participants on site. To make an appointment, please contact Ron Hebert, SVP Marketing, at rhebert@covalon.com.
Conference details
Dates: Tuesday, June 27 – Friday, June 30, 2023
Venue: Hyatt Regency St. Louis at The Arch (315 Chestnut Street, St. Louis, Missouri 63102)
Registration: https://oley.org/event/Oley2023
For healthcare providers who are not able to attend the conference but are interested in learning more about Covalon’s solutions, visit www.covalon.com or follow Covalon on LinkedIn, Facebook, Instagram or Twitter.
About Covalon
Covalon Technologies Ltd. is a patient-driven medical device company, built on the relentless pursuit to help the most vulnerable patients have a better chance at healing. Through a strong portfolio of patented technologies and solutions for advanced wound care, infection prevention, and medical device coatings, we offer innovative, gentler, and more compassionate options for patients to heal with less infections, less pain, and better outcomes. Our solutions are designed for patients and made for care providers. Covalon leverages its patented medical technology platforms and expertise in two ways: (i) by developing products that are sold under Covalon’s name; and (ii) by developing and commercializing medical products for other medical companies under development and license contracts. The Company is listed on the TSX Venture Exchange, having the symbol COV and trades on the OTCQX Market under the symbol CVALF. To learn more about Covalon, visit our website at www.covalon.com.
Neither the TSX Venture Exchange nor its Regulation Services Provider (as that term is defined in the policies of the TSX Venture Exchange) accepts responsibility for the adequacy or accuracy of this release.
This news release may contain forward-looking statements which reflect the Company's current expectations regarding future events. The forward-looking statements are often, but not always, identified by the use of words such as "seek", "anticipate", "plan, "estimate", "expect", "intend", or variations of such words and phrases or state that certain actions, events, or results “may”, “could”, “would”, “might”, “will” or “will be taken”, “occur”, or “be achieved”. In addition, any statements that refer to expectations, projections or other characterizations of future events or circumstances contain forward-looking information. Statements containing forward-looking information are not historical facts, but instead represent management’s expectations, estimates, and projections regarding future events. Forward-looking statements involve risks and uncertainties, including, but not limited to, the factors described in greater detail in the “Risks and Uncertainties” section of our management’s discussion and analysis of financial condition and results of operations for the year ended September 30, 2022, which is available on the Company’s profile at www.sedar.com, any of which could cause results, performance, or achievements to differ materially from the results discussed or implied in the forward-looking statements. Investors should not place undue reliance on any forward-looking statements. The forward-looking statements contained in this news release are made as of the date of this news release, and the Company assumes no obligation to update or alter any forward-looking statements, whether as a result of new information, further events, or otherwise, except as required by law.

View source version on businesswire.com: https://www.businesswire.com/news/home/20230627972227/en/