Richard (Rick) Mills
Ahead of the Herd
As a general rule, the most successful man in life is the man who has the best information
Minimally invasive therapy is becoming more and more common in hospitals. These procedures are performed through tiny incisions (instead of one large opening) or our body’s natural orifices.
A long, thin tube with a miniature camera attached at the end, an endoscope, is passed into the body. Images from the endoscope are projected onto monitors in specialized examination rooms and O.R.’s (operating room) so doctors and surgeons can get a magnified view.
There are many different types of endoscopes (Cystoscopes, Bronchoscopes, Gastroscopes, Laporascopes and others) and depending on the site in the body, and the type of procedure, endoscopy may be performed by a doctor or a surgeon, and the patient may be fully conscious or under general anesthetic.
Endoscopies are commonly performed in the diagnosis of cancer; for taking samples of tissue, called biopsies to find out whether it is cancerous, as well as for complete excision - the cutting out - of suspicious lesions.
Endoscopes are also used in laparoscopic surgery in which a small puncture is made, usually in the navel, through which a camera is inserted. This allows the doctor to examine the abdominal and pelvic organs on a video monitor connected to the tube.
Laparoscopes are utilized in surgery to visualize various organs and tissues within the body during surgical removal and to avoid damage to adjacent organ and vascular systems and other critical structures. Laparoscopy is less invasive than regular open abdominal surgery.
Because of the endoscope biopsies of the intestines or lungs can be done without the need for major surgery.
Patients, physicians, providers, and payers have wholeheartedly embraced minimally invasive therapy for many reasons:
- Minimally invasive therapy obviates the need for major open-surgery procedures.
- Minimally invasive therapy eliminates the need for general anesthesia.
- Minimally invasive therapy produces much less of the sequelae (a condition that is the consequence of a previous disease or injury) of open surgery procedures.
- Minimally invasive therapy leaves minute scars versus open-surgery procedures.
- Minimally invasive therapy results in shorter hospital stays and reduced outpatient treatments.
- Minimally invasive therapy results in a much more rapid return to normal activity.
- Reductions in length of hospitalization and the ability to return to work much sooner are economically attractive.
The endoscope is the main or central technological component of minimally invasive therapy.
Good news - minimally invasive therapy, and the evolution of the endoscope, are about to be fast forwarded. Interdisciplinary and inter-institutional forces have been working together to bring forth a new generation of endoscopy technology.
But before we get to new technological advances let’s look at current endoscope technology as it involves cancer.
White Light and Cancer
White light is the standard convention and it’s what’s commercially available in all endoscope devices manufactured today. White light has been utilized in endoscopes for decades to guide the physician and surgeon so they can see cancerous growths that protrude above an organs surface, do biopsies and remove suspicious growths.
White light is comprised of energy in the form of electromagnetic radiation that vibrates at many different wavelengths. Wavelengths between 390 nm and 780 nm are visible to the human eye and produce the different colors of the spectrum.
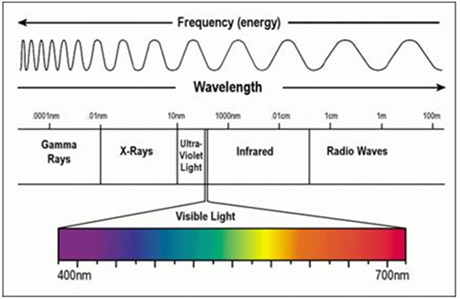
White light has limitations in visualizing certain cancer types because:
- White light cannot pass through tissue or blood
- White light cannot illuminate tumors beneath the skin surface.
- White light is not effective in visualizing the borders of the tumor to determine where it begins and ends (the margins), especially after the initial removal of the main mass. If the surgeon does not remove all the cancerous growth, and a few cancerous cells remain, the tumor can grow back and spread, or metastasize to other parts of the body
- Malignant and premalignant tumors that are flat or very small may look similar to normal tissues. As a result, a physician may not be able to identify some aggressive cancers. In order to be safe, they may collect random and repeat biopsies as the only possible way to ensure that cancer is not missed in high-risk patients
To summarize, white light has visualization limitations for all cancer types because white light cannot pass through tissue or blood and cannot illuminate tumors beneath the skin surface. White light is also not effective in visualizing the borders or margins of the tumor to determine where it starts and ends especially after the initial removal of the main mass.
Blue Light
Because of the limitations with using white light for visualizing cancers, various companies have begun using blue light (white light with blue filter) in conjunction with chemical tumor targeting/imaging agents. This improved technology introduces a red fluorescence to the tumor and has improved the ability to visualize cancers and margins.
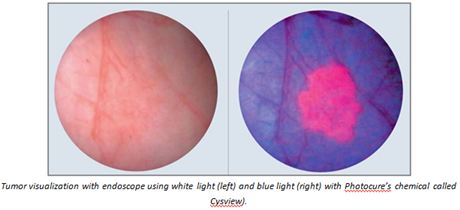
Unfortunately these chemical agents can cause various adverse effects - including anaphylaxis shock and hypersensitivity reactions - with repeated usage at the high doses currently required for visualization. The FDA has limited use of these chemical tumor targeting/imaging agents to just once per patient. Doctors and surgeons cannot repeatedly examine a patient using these chemical imaging agents. This creates a huge problem treating patients with multiple tumors and those with recurrent tumors.
Red Light
Red light requires specialized laser light sources, ultrasensitive cameras and a unique optical design. Currently no commercial instruments are available using red light.
The Unmet Need
What is acutely needed right now, across the entire spectrum of the endoscope imaging space/sector, is an ultrasensitive system that uses white light while simultaneously using other wavelengths of light to visualize all tumors, and one that requires only a fraction of the chemical imaging agent so as to reduce the toxicity allowing multiple usage in patents.
Future endoscopes should also have more advanced cancer detection technologies so that ultimately no chemical imaging agents would be necessary.
These future tools should provide ultrasensitive and advanced imaging capabilities and the system should be capable of being used in a physician’s office or clinic for diagnosis, in the operating room and ambulatory surgical center.
Imagin Medical CSE – IME
Imagin Medical is a biotechnology company founded to commercialize an ultrasensitive, next generation imaging technology for extremely accurate visualization of cancers.
IME believes it will radically improve the way physicians detect cancer through the use of endoscopes to image and visualize cancer and/or cancer markers.
Imagin is developing systems that will potentially enable physicians to accurately detect cancer with minimal use of, or no toxic contrast agent, and remove all cells completely during the first procedure. This technology, after having being proven successful in the operating room, will greatly reduce the chances of cancer recurrence and allow safe, multiple follow-up screenings for patients that can be performed during routine monitoring by physicians in an outpatient setting.
Imagin’s next generation imaging technology was invented and developed by Dr. Stavros Demos. Dr. Demos invented a combination optical/laser technology that uses blue light and near-infrared fluorescence for the accurate visual detection of cancer. Dr. Demos worked with the Lawrence Livermore National Laboratory (LLNL) and the U.C. Davis NSF Center for Biophotonics Science and Technology for more than eight years creating this technology and demonstrating its proof of principle.
Imagin Medical is partnering with LLNL and UC Davis to complete the clinical evaluations that will support the Company’s FDA submission. Dr. Demos is Imagin Medical’s Technical Scientific Advisor and Imagin holds the exclusive license to this intellectual property.
This exclusive license includes the three issued patents and two pending patent applications on technology related to exclusive spectroscopic imaging for cancer and other medical applications.
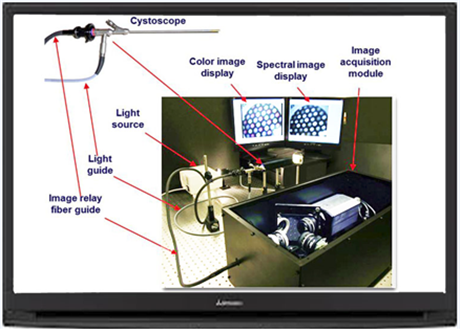
Imagin Medical’s advanced ultrasensitive imaging technology is based upon improved optical designs and components, and advanced light sensors. The results are:
- Increased sensitivity and specificity for the detection of cancers and even premalignant lesions.
- A potential decrease in cancer recurrence due to the ability to completely remove tumor tissues along with the cancerous cells in the margins.
- A significant commercial advantage to Imagin’s imaging technology because of its adaptability to all endoscopes that are currently on the market.
- Easy adoption of Imagin’s two ultrasensitive imaging designs for use in multiple other applications where endoscopy imaging is currently utilized.
i/Blue Imaging System
A unique optical design and targeted imaging dyes have been combined to create the i/Blue Imaging System, one that far surpasses the imaging capability of blue light systems on the market today.
This new technology not only brings cancer imaging to a new level, but it should also reduce the amount of contrast agents required by 99%. This considerably smaller dose increases patient safety and means that physicians will be able to perform multiple procedures, not only in the O.R., but also in the less expensive office setting. The high sensitivity of the system will enable the detection of signal by the bladder tumor in minutes versus one hour, the current prescribed retention time of the agent before fluorescence imaging examination of the patient, thus improving the efficiency of both the O.R. and the physician’s office.
Additionally, physicians using the i/Blue Imaging System will have the advantage of Imagin’s dual imaging technology. The surgeon and O.R. staff will no longer need to “switch” back and forth between the white light and the blue light images because the i/Blue Imaging System blends both imaging modes into one image.
i/Red Ultrasensitive Imaging System
Imagin’s second proprietary imaging system uses red light and requires no contrast agents at all. The i/red system uses the fluorescence produced by the body and tumor when illuminated by the red laser light.
The company believes there will be no need for chemical imaging agents, and therefore physicians will be able to perform a diagnosis in the office for cystoscopy (diagnosis) and, if a tumor is detected in an early stage, will be able to remove it at the same time and eliminate the need and costs associated with the O.R.
Without the threat of adverse side effects from chemical agents, the physician will be free to conduct multiple follow-up visits with individual patients during routine checkups in the office setting.
In the operating room, the i/Red Imaging System will enable the effective sampling of biopsies that cannot be seen under white light and equally important, enhance the visibility of tumor margins to be sure that all the cancerous cells are removed.
Unlike other technologies, red light imaging allows deeper tissue imaging without artifacts from vasculature. The i/Red System also will provide dual imaging technology, adding the same convenience as i/blue for the surgeon and O.R. staff.
Imagin’s i/Red Imaging System will dramatically broaden the market to all cancer specialists using any type of scopes.
Competitive Advantages
Imagin Medical’s advanced ultrasensitive imaging technology will lead the marketplace in illumination of cancerous cells and provide an improved surgical outcome as a result of an improved detection and resection, which will lead to more adequate patient management and follow-up.
Competitive Advantages:
- Can be seamlessly adapted to any type of endoscopic or other type of imaging device commercially available.
- Based upon improved optical designs and components, and advanced light sensors. The result is increased sensitivity and specificity for the detection of cancers, including premalignant lesions.
- Will decrease bladder cancer recurrence due to the ability to completely remove tumor tissues along the margins.
- Will add significant ease-of-use for the surgeon and staff in the O.R. because of the dual imaging capability.
- Use less than 1 percent of the toxic chemical currently (such as Cysview) used per test. Cysview is already FDA approved.
- Imagin Medical has an ultrasensitive red light endoscopy system that requires no chemical imaging agents.
- Because there is no need for chemical imaging agents, this system can be used in a physician’s office or clinic for cystoscopy (diagnosis), and in the operating room (O.R.) or ambulatory surgical center for tumor removal or resection.
- Imagin Medical has patents covering these imaging systems that are estimated to be at least 1000 times more sensitive in tumor detection than any other devices currently in the marketplace.
- Provide improved detection of cancer superior to what is currently in the market place.
- The instrumentation involved does not come into contact with the patient, thus significantly reducing regulatory requirements and associated expenditures.
- The white light and fluorescence images are recorded and displayed simultaneously providing an effective real time navigation tool that can be farther enhanced using processing (such as overlapping and pseudo-coloring) of the two principal images.
Endoscope Market
Over the last 50 years, there has been an intense effort worldwide to find new methods to detect and treat cancer.
The demand for endoscopy as a tool in cancer detection has been increasing significantly because of the growing preference for minimally invasive surgeries, which reduce patients’ pain, speed recovery and reduce the overall costs to the healthcare system.

The other factors that are driving the growth of the global endoscopy equipment market include:
- Favorable reimbursement in select regions
- Aging population
- Increasing prevalence of diseases that require endoscopy procedures
Bladder Cancer
The Company’s initial commercial application will be its i/Blue Imaging System for the detection and effective removal of bladder cancer in the operating room or O.R.
According to the National Cancer Institute (NCI) bladder cancer (a US$500 million market) is the sixth most common cancer in the United States and the third most common cancer in men. It is estimated that approximately 577,400 people are currently living with bladder cancer in the United States with over 72,000 new cases being diagnosed annually (380,000 worldwide with 150,000 deaths per year), generating over 1,000,000 physician consultations per year. Approximately 16,000 individuals in the U.S. will die from the disease in 2015.
The company believes to be successful it must focus on one surgical specialty at a time. Bladder cancer is the company’s initial focus because it is the most expensive cancer to treat in the US. Low grade non-muscle bladder cancer has a reoccurrence rate of 40% while high grade non-muscle bladder cancer has a reoccurrence rate of 70%. These are some of the highest reoccurrence rates of all cancers and is why patients require several checkups (involving the use of endoscopes) per year for many years after initial surgery.
Once established in the bladder cancer market, and within the urology community, Imagin will aggressively move into other surgical disciplines.
Regulatory
The Food and Drug Administration (FDA) classifies medical devices into one of the three categories based on the risks associated with each device.
Class I: Devices deemed to be the lowest risk and therefore subject to the lowest regulatory controls.
Class II: Devices are higher risk and require greater regulatory controls to provide reasonable assurance of safety and effectiveness. For example, a cardiac monitor and a nebulizer are Class II devices.
Class III: Devices are generally the highest risk and must typically follow a Premarket Approval (PMA) route. For example, replacement heart valves are classified as Class III devices; a powered toothbrush and dental floss are Class I devices.
Fluorescent drugs are already approved by the FDA for use with existing, approved endoscopic light sources. Since Imagin’s labeling will not vary from the already FDA approved use of these contrast agents and blue light lasers and light sources already exist (and are being used in the market for various medical specialties) Imagin likely will not need to prove its underlying technology, that being:
1. The use of blue light to aid in imaging tumors using imaging agents.
2. The use of red light to detect auto-fluorescence and identify tumor margins during its clinical trial.
The company believes the FDA will require Imagin’s study to focus on whether their combined image method performs as well as, or better than, existing technologies to demonstrate that Imagin’s systems are as safe and effective as existing technologies for their labeled intended uses.
Preliminary testing was performed in vivo (studies and trials performed inside the living body) in 21 patients undergoing transurethral resection of bladder tumors at the UC Davis Medical Center, a well-respected cancer facility, with excellent results.
The company expects a Class III regulatory path. A 6-12 month process for FDA approval is an aggressive but possible timeframe.
Management Team & Advisors
E. James Hutchens, Chief Executive Officer - Jim is a proven entrepreneur with over 30 years of experience in general and marketing management in the medical device industry. As the founder and CEO of Microsurge Inc., a venture-backed, minimally invasive surgery company, he assembled a management team, guided the company’s products through the regulatory process, hired a sales and marketing team, drove revenues to an annual run rate of over $10 million and sold the company. As the founder and CEO of Choice Therapeutics, an advanced wound care company, Mr. Hutchens implemented similar tactics and with revenue of $2 million sold the company to Alliqua Biomedical a NASDAQ list company. At Microvasive Endoscopy, a division of Boston Scientific, revenues rose from $300,000 to over $20 million during his tenure as Vice President, Marketing and Sales.
Thom McMahon, Vice President of Sales - Thom has been involved in medical device sales and marketing for over 35 years. Thom is currently the owner and president of Urolaze, an independent urology focused distribution company. Thom’s team is responsible for Siemens C-Aim and lasers as well as American Medical Systems prostate lasers and other products. He has spent over 25 years with Karl Storz in various sales and marketing positions and was the number one sales representative for over 10 years.
Michael G. Vergano, Vice President Operations - Michael has been president of The Harvest Group Inc. since 1998, where he has provided project management, product development, packaging, quality systems, operations, manufacturing engineering, and system/process design and validation services for start-ups as well as major medical corporations. His medical device experience includes the gynecology, laparoscopic surgery, GI endoscopy, dental imaging and implants, wound care, cardiology, urinary incontinence and orthobiologic regenerative technologies markets.
Stephen Ruggles, Vice President, Regulatory - Stephen has over 30 years of experience in domestic and international regulatory affairs, operations management, manufacturing quality assurance, R&D, and supply chain management for early stage companies and large multinationals. As Director of QA / RA for Cambridge Endoscopic Devices, Mr. Ruggles led the effort to earn and maintain ISO 13485 registration, CE Mark, and compliance with Japan's jPAL.
Stavros G. Demos, Ph.D., Technical Scientific Advisor – Dr. Demos is a scientist at Lawrence Livermore National Laboratory in the Physical and Life Sciences Directorate and scientific staff at the National Science Foundation Center of Biophotonics Science and Technology at UC Davis. He received his B.S. in physics from the University of Ioannina, Greece, and his Ph.D. in physics in 1993 from the City University of New York. His research interests are concentrated in the field of biomedical optics and laser-materials interactions. He is working to develop instrumentation for in vivo detection of disease and monitoring of therapeutic intervention. In the field of defect characterization, he is working on optical materials for tunable solid-state and high-power laser systems. Dr. Demos has published over 115 articles in refereed journals, holds 20 patents and was elected Fellow of the American Physical Society.
Ralph DeVere White, M.D., Medical Advisor - Dr. deVere White is associate dean for cancer programs at UC Davis School of Medicine, Director of the UC Davis Comprehensive Cancer Center where he is also a Professor of Urology. He received his medical degree from Dublin University in Dublin, Ireland, and completed an internship and residencies in surgery and urology at St. Vincent’s Hospital in Dublin and at Duke University Medical Center in Durham, NC. The author of more than 300 peer-reviewed scientific articles and book chapters, Dr. deVere White serves on the editorial boards of six international scientific journals. His current research efforts focused on the molecular events that govern the response of prostate and bladder cancer to targeted therapies, the bio-molecular mechanisms that make some prostate cancers more virulent than others, and new methods of diagnosing and treating prostate cancer. He is the immediate past president-elect of the Society of Urologic Oncology, a member of the Clinical Society of Genitourinary Surgeons and an elected member of the prestigious American Association of Genitourinary Surgeons. He has been named multiple times as one of "The Best Doctors in America" and is one of four urologists serving as a volunteer consultant to the American Cancer Society's on-line "Experts Answers" bulletin board for questions about prostate cancer.
Conclusion
Current endoscopy instruments have well-documented problems associated with the limited ability to distinguish cancer from normal tissue.
Imagin’s strategy is to set a new “standard of care” in detecting cancer by introducing game-changing disruptive advances in endoscope technology with its i/Blue and i/Red combination optical/laser systems.
Imagin Medical CSE - IME, and their significantly improved endoscope technology, need to be on your radar screen.
Is advanced endoscopy on your screen?
If not, it should be.
Richard (Rick) Mills
Richard lives with his family on a 160 acre ranch in northern British Columbia. He invests in the resource and biotechnology/pharmaceutical sectors and is the owner of Aheadoftheherd.com. His articles have been published on over 400 websites, including:
WallStreetJournal, USAToday, NationalPost, Lewrockwell, MontrealGazette, VancouverSun, CBSnews, HuffingtonPost, Beforeitsnews, Londonthenews, Wealthwire, CalgaryHerald, Forbes, Dallasnews, SGTreport, Vantagewire, Indiatimes, Ninemsn, Ibtimes, Businessweek, HongKongHerald, Moneytalks, SeekingAlpha, BusinessInsider, Investing.com, MSN.com and the Association of Mining Analysts.
Please visit www.aheadoftheherd.com – We’re telling you things everyone else doesn’t already know.
Moderated investor friendly forums - Ahead of the Herd is powered by Community Intelligence.
Free highly acclaimed newsletter featuring today’s investable junior resource companies.
If you are interested in sponsoring Richard’s site please contact him for more information, rick@aheadoftheherd.com
***
Legal Notice / Disclaimer
This document is not and should not be construed as an offer to sell or the solicitation of an offer to purchase or subscribe for any investment.
Richard Mills has based this document on information obtained from sources he believes to be reliable but which has not been independently verified.
Richard Mills makes no guarantee, representation or warranty and accepts no responsibility or liability as to its accuracy or completeness. Expressions of opinion are those of Richard Mills only and are subject to change without notice. Richard Mills assumes no warranty, liability or guarantee for the current relevance, correctness or completeness of any information provided within this Report and will not be held liable for the consequence of reliance upon any opinion or statement contained herein or any omission.
Furthermore, I, Richard Mills, assume no liability for any direct or indirect loss or damage or, in particular, for lost profit, which you may incur as a result of the use and existence of the information provided within this Report.
Richard owns shares of Imagin Medical CSE - IME
Imagin Medical TSX.V - IME is a sponsor of Richards site aheadoftheherd.com