Reliq Health Technologies is initiating a trial of its remote patient monitoring system with The Feldman Institute in Louisiana, the fourth program for the company's innovative technology.
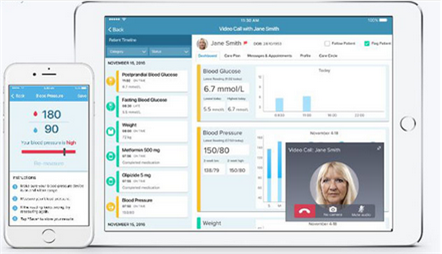
Reliq Health Technologies Inc. (RHT:TSX.V), a Vancouver-based technology company that is developing solutions for the community-care market, announced on Dec. 1 that it has begun enrolling patients in a pilot test program at The Feldman Institute in Baton Rouge, Louisiana. According to the company, the pilot "will evaluate the use of Reliq Health's technology with patients who have been discharged home after interventional pain management surgery, or have returned to their homes between treatments at The Feldman Institute."
"We are very pleased to be enrolling patients and launching our pilot of the Reliq Health platform with The Feldman Institute," said Dr. Lisa Crossley, CEO of Reliq Health Technologies, Inc. "Our virtual care solution allows patients to receive high-quality follow-up care from the comfort of their own homes. Patients can communicate with their care team at The Feldman Institute using Reliq's cloud-based secure messaging, videoconferencing and virtual visits. The Reliq Health cloud provides access to patient education materials and instructional videos, empowering patients and their families to actively participate in their own care at home."
Patients receive a technology suite that includes a two-way technology hub, proximity sensors and wearable biometric devices, Dr. Crossley told The Life Sciences Report. The system tracks vital signs, reminds the patient to take medications and knows if the patient fails to do so, and alerts family and health care providers to prevent further escalation of the disease. The information is stored in a secure cloud and allows the patient's healthcare team to collaborate and avoid costly redundancies.
The pilot with The Feldman Institute joins existing pilots with the Sacred Heart Health System in Florida, a healthcare network consisting of three hospitals and a large network of physicians; NHS England; and a white-label contract with the City of San Antonio, Texas, for the development of a proactive, wellness-focused mobile app for use by its residents.
NHS England is using Reliq's remote patient monitoring system with patients with diabetes and congestive heart failure. According to Internet of Business, NHS England has over 15 million patients with at least one chronic condition, and has estimated that self-care improvements could save £584 million ($725 million) by 2021.
In the U.S., chronic conditions comprise more than 80% of healthcare costs in 2015, according to Reliq, costing $2.6 trillion. Medicare and Medicaid now fine hospitals for "preventable readmissions"—in 2015, 76% of hospitals incurred Medicare/Medicaid readmission penalties—so hospitals have the financial incentive to actively avoid readmissions.
Michael Berger, the president of Technical420 and a former Raymond James analyst, told MoneyShow.com that he is "favorable on Reliq due to its ability to disrupt a multi-billion-dollar market, its strong management team that continues to execute on its initiatives, its four pilot programs, and its attractive valuation. . .Reliq Health offers a very attractive opportunity for investors after it secured several pilot programs and this provides the market with a visible path for growth."
Want to read more Life Sciences Report articles like this? Sign up for our free e-newsletter, and you'll learn when new articles have been published. To see recent articles and interviews with industry analysts and commentators, visit our Streetwise Interviews page.
Disclosures:
1) Patrice Fusillo compiled this article for Streetwise Reports LLC and provides services to Streetwise Reports as an employee. She owns, or members of her immediate household or family own, shares of the following companies mentioned in this article: None. She is, or members of her immediate household or family are, paid by the following companies mentioned in this article: None.
2) Reliq Health Technologies Inc. is a billboard sponsor of Streetwise Reports. Streetwise Reports does not accept stock in exchange for its services. The information provided above is for informational purposes only and is not a recommendation to buy or sell any security.
3) Comments and opinions expressed are those of the specific experts and not of Streetwise Reports or its officers.
4) The article does not constitute investment advice. Each reader is encouraged to consult with his or her individual financial professional and any action a reader takes as a result of information presented here is his or her own responsibility. By opening this page, each reader accepts and agrees to Streetwise Reports' terms of use and full legal disclaimer. This article is not a solicitation for investment. Streetwise Reports does not render general or specific investment advice and the information on Streetwise Reports should not be considered a recommendation to buy or sell any security. Streetwise Reports does not endorse or recommend the business, products, services or securities of any company mentioned on Streetwise Reports.
5) From time to time, Streetwise Reports LLC and its directors, officers, employees or members of their families, as well as persons interviewed for articles and interviews on the site, may have a long or short position in securities mentioned. Directors, officers, employees or members of their families are prohibited from making purchases and/or sales of those securities in the open market or otherwise during the up-to-four-week interval from the time of the interview or article until after it publishes.