At the forefront of the next wave of cannabis commercialization, is a German-focused company with headquarters in Vancouver,
XPhyto Therapeutics Corp. (CSE: XPHY; FSE: 4XT), that is building a much-coveted cannabis R&D cultivation facility in a converted nuclear bunker and owns an additional EU GMP laboratory and cannabis import licences in Germany.
Though a newcomer to the capital markets, it has made some major strides over the past few weeks, having already launched some major developmental and operational initiatives in Germany – an affluent, populous nation that has an ageing population and which is projected to become the world’s largest medical cannabis market.
 (XPhyto is accelerating science-based therapeutic validation for medical cannabis.)
(XPhyto is accelerating science-based therapeutic validation for medical cannabis.)
XPhyto is focused on the most advanced applied science of cannabinoid plant analytics. Its phytochemical analysis and processing technology yields hyper-refined isolates for use in clinical trials, therapeutic treatments and pharmaceutical drug delivery systems. It also provides tools and methods for commercial testing and certification services.
Based on current operations in Canada and Germany, XPhyto’s CEO and Director Hugh Rogers says that the long-term vision “is to build one of the largest distribution networks in Germany for medical and CBD products, as well as clinical validation in a more conventional pharmaceutical industry.”
In effect, XPhyto’s ultimate outlook potentially encompasses the entire global market for advanced pharmaceutical and therapeutic cannabis applications.
In light of the recent scandal over tainted vape pen cartridges, investors, medical patients and adult-use users are all looking for the innovation of safer delivery systems to win back their trust. Hence, the recent announcement of XPhyto’s acquisition of Vektor Pharma TF (Therapeutic Film) GmbH in Germany is a true game changer.
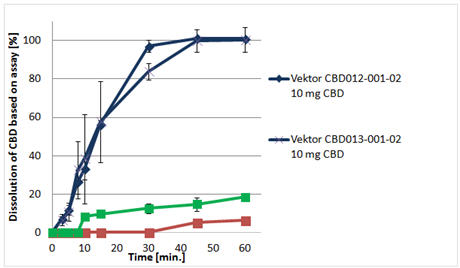 (These results show the superior dissolution abilities of Vector’s CBD formulations and oral delivery system, compared to CBD softgel capsules and chewing gum.)
(These results show the superior dissolution abilities of Vector’s CBD formulations and oral delivery system, compared to CBD softgel capsules and chewing gum.)
Vector has over ten years in the design, testing and manufacture of thin-film drug delivery systems, particularly transdermal patches and sublingual (oral) strips for the clinical management of pain. Vector has a global profile, with more than half its current revenue coming from non-EU locations including Asia and North America.
Additionally, Vektor holds a complete slate of valid narcotics licences pursuant to EU GMP certification and other governing regulations. These licences include authorizations related to conventional and cannabis-related prescription medications, allowing Vektor to import, manufacture and perform analytics, testing and production across a wide range of novel treatment applications.
 (Vektor manufacturing facilities producing therapeutic film.)
(Vektor manufacturing facilities producing therapeutic film.)
According to XPhyto CEO Rogers, “The Vektor transaction will accelerate XPhyto’s medicinal cannabis import into Germany and its drug delivery expertise, both of which are a critical part of our near-term revenue generation strategy. Further, with vape-based delivery systems now associated with significant potential health risks, XPhyto is extremely pleased to combine assets and expertise with Vektor, a specialist in thin-film drug delivery systems.”
Germany Leads EU in Cannabis Normalization
Germany legalized medical marijuana in March 2017. Cannabis consultant and lawyer Kai-Friedrich Niermann notes, “The market for medical cannabis [in Germany] has grown enormously since the change of legislation. Total sales will reach €100 million by the end of 2019, with the share of finished drugs and cannabis preparations permanently exceeding unprocessed cannabis flower.”
Furthermore, according to a recent Prohibition Partners report, “by 2024, over a million German patients will have access to medical cannabis, and by 2028 the medical market alone will be worth €7.7 billion.” So far in 2019, 60% of German medical cannabis prescriptions have been reimbursed by insurance companies, pointing the way to even higher rates of adoption going forward.
With Germany well-established as the leader in European cannabis normalization, XPhyto is ideally positioned to enter other EU jurisdictions as medical cannabis regulations evolve elsewhere on the continent. Industry standards defined by the German experience are likely to influence the shape of legislation and regulation in other countries, and XPhyto will be at the centre of that movement.
Becoming A Leading Cannabis Innovator in Germany
 (XPhyto’s retrofitted medical cannabis R&D facility near Munich.)
(XPhyto’s retrofitted medical cannabis R&D facility near Munich.)
In addition to XPhyto’s strategic acquisition of Vektor Pharma TF, the company is making good use of its eye-catching underground laboratory in Bavaria. When it comes to meeting the highly exacting security levels required for licencing by the German Federal Institute for Drugs and Medical Devices, XPhyto’s repurposed nuclear bunker will be fully compliant with EU-GMP standards.
This critical validation is particularly important because Bunker Pflanzenextrakte (Plant Extracts) GmbH – which is 100%-owned by XPhyto and located in the Munich West Commercial Airport complex – has a unique licence to cultivate and derive extractions from up to 70 different strains of cannabis for scientific purposes.
It’s worth noting that only industry titans Aurora Cannabis and Aphria Inc. also have the privilege of also growing a multitude of strains in Germany too, albeit for the sale of flower to the German government, rather than for R&D and extraction purposes.
In conjunction with this licence, Bunker has an exclusive cannabis research and development agreement with the world-renowned Technical University of Munich (TUM), Faculty of Chemistry, to isolate and assess various cannabis derivatives for potential commercial applications.
Also, it has an exclusive agreement with the TUM School of Life Sciences, Brewing and Beverage Technology, to develop refined bioactive ingredients for potential commercialization of new cannabis-derived foods, dietary supplements and beverages.
Canadian Operations Offer Top-Level R&D and Testing Capabilities
XPhyto Laboratories Inc. is a 100%-owned subsidiary operating through two exclusive 5-year agreements around cost and profit sharing in collaboration with the Faculty of Pharmacy at the University of Alberta.
These agreements are connected with the cannabis research and intellectual property of internationally renowned Dr. Raimar Löbenberg, founder and Director of the university’s Drug Development and Innovation Centre, and President of the Canadian Society of Pharmaceutical Sciences.
These arrangements will generate two separate but complementary business lines for XPhyto Labs. One will provide IP and advanced equipment to formulate and manufacture pharmaceutical-grade extracts and isolates for therapeutic validation, pilot studies and clinical trials.
The other will operate a commercial-grade analytical lab for the testing of cannabis and other plant-based medicines (identifying cannabinoid and other bioactive ingredients, as well as contaminants such as heavy metals, pathogens and pesticides, etc.).
While R&D and manufacturing operations target medium and longer-term results, the testing laboratory will satisfy an immediate and acute need. Access to testing and certification services has always been one of the main bottlenecks for producers trying to get product to consumers.
In addition, vaping-related products are now the focus of intense health concerns, and Cannabis 2.0 is kicking in with a wide array of new products trying to reach the market.
The resulting spike in demand for testing and certification services should support a robust near-term revenue stream for XPhyto Labs. And because this is an essential service for all industry participants, from licenced producers to distributors to government agencies, long term revenue results should provide XPhyto with a consistent and stable baseline cash flow.
Investment Summary
As Germany brings medical cannabis into the healthcare mainstream, it will in all likelihood continue to be methodical, rigorous, and tightly regulated in its approach. XPhyto’s world-class R&D work fits in perfectly with this model of commercial development. In fact, without the kind of clinical progress XPhyto can achieve, there won’t be much of an evolution in the development of innovative downstream cannabis products in Europe’s largest and most advanced cannabis marketplace.
With few exceptions, mainstream medical practitioners will not prescribe treatments based on anecdotal therapeutic value using unpredictable dosage delivery systems. The value of cannabis-based medical treatments on a widespread basis will only come about as a result of refined active ingredient isolation and dosage delivery technologies. Even the clinical trials necessary to prove specific cannabinoid treatment indications cannot be done without the successful application of advanced phyto-pharmaceutical formulation and manufacturing.
This reality shows that XPhyto is investing in exactly the right part of the market cycle, where all of the future exponential growth for medical cannabis is expected to be generated. The spill-over benefits of this medical technology into the food, beverage and dietary supplement markets promise even greater returns on capital.
With expanding near-term operating revenues and the unsurpassed expertise of its R&D and manufacturing partners, as well as its own operating subsidiaries, we don’t expect XPhyto to remain under the radar for much longer.
ABOUT THE AUTHORS: Daniel Brooks is Senior Editor of CannabisCapitalist. Marc Davis has a deep background in the capital markets spanning 30 years, having mostly worked as an analyst and stock market commentator. He is also a longstanding financial journalist. Over the years, his articles have also appeared in dozens of digital publications worldwide. They include USA Today, CBS Money Watch, Investors’ Business Daily, the Financial Post, Reuters, National Post, Google News, Barron’s, China Daily, Huffington Post and AOL.